武汉华美生物工程有限公司CUSABIO®品牌商
15 年
手机商铺
- NaN
- 0
- 0
- 2
- 2
推荐产品
公司新闻/正文
ACVR1C/ALK7:代谢性疾病与肿瘤治疗中的新兴靶点
266 人阅读发布时间:2026-01-12 14:39
ACVR1C/ALK7作为TGF-β超家族的关键成员,近年来因其在代谢调控与肿瘤进展中的双重角色而备受关注。它不仅在脂肪组织分化、胰岛素敏感性与能量平衡中发挥重要作用,也在多种实体瘤中表现出抑制或促进肿瘤生长的“情境依赖性”。本文系统梳理了ACVR1C/ALK7的分子机制、信号通路及其在代谢性疾病与肿瘤中的功能,以期对您的相关研究有所帮助。
1. ACVR1C/ALK7研究背景与生物医学意义
2. ACVR1C/ALK7的分子特征、结构基础与配体识别
3. ACVR1C/ALK7介导的信号转导通路
4. ACVR1C/ALK7 在疾病发生发展中的作用
5. ACVR1C/ALK7靶向药物最近研究进展
6. ACVR1C/ALK7相关研究工具
1. ACVR1C/ALK7研究背景与生物医学意义
ACVR1C(Activin receptor-like kinase 7,ALK7)是TGF-β超家族中的I型受体成员,具有典型的受体丝氨酸/苏氨酸激酶结构域及GS调控区,其功能依赖于与II型受体形成复合体并介导下游信号级联。早期克隆与表达谱研究显示,ACVR1C在胚胎发育及成体组织中呈现显著的时空特异性表达,这一特征为其在发育调控与成人组织稳态中的作用提供了基础证据。尽管其激酶核心与ALK4/ALK5高度保守,胞外区的微小差异却显著影响配体亲和性与信号输出强度,使ACVR1C在功能上表现出独特性。
在信号转导层面,ACVR1C主要激活Smad2/3介导的经典TGF-β样通路,同时在特定细胞环境下可并行触发MAPK、PI3K/AKT等非经典通路,从而实现对细胞增殖、分化、迁移与代谢的综合调控。越来越多的证据表明,其信号输出高度依赖于配体类型、受体复合体构成以及细胞内调节因子(如I-Smad与E3泛素连接酶),这为解释ACVR1C在不同生理与病理情境下产生相反效应提供了机制基础。然而,现有研究多依赖体外过表达体系或广谱抑制剂,限制了对ACVR1C内在功能及其与其他I型受体交互关系的精确解析,凸显了发展选择性工具与条件性在体模型的必要性。
功能研究已将ACVR1C与胚胎发育、脂肪组织稳态和能量代谢紧密联系。基因敲除与发育模型提示其参与干细胞命运决定与器官发生,而在成体代谢组织中,ACVR1C通过抑制脂肪前体细胞增殖并促进分化,影响胰岛素敏感性与能量平衡,使其成为肥胖与2型糖尿病研究的重要关注点。相对地,在肿瘤与纤维化研究中,ACVR1C的作用呈现明显的双重性:既可在部分肿瘤背景下发挥抑制作用,也可能在特定微环境中促进侵袭与迁移,反映出细胞背景与配体谱对其信号输出的决定性影响。由于人类遗传与临床关联证据仍有限,如何从基因层面确立ACVR1C与疾病表型之间的因果关系,仍是该领域的重要挑战。
2. ACVR1C/ALK7的分子特征、结构基础与配体识别
2.1 基因结构、蛋白构象与组织表达特征
ACVR1C属于TGF-β超家族I型受体,包含胞外配体结合区、单次跨膜螺旋及胞内GS区与激酶结构域。其最初作为孤儿受体被克隆,随后被确认可作为Nodal与Activin类配体的I型受体 [1]。结构与结合研究表明,Nodal与Cripto、ALK4/ALK7之间形成多分子复合体,其关键氨基酸位点决定了配体选择性 [2]。值得注意的是,Activin类配体具有双特异性:在ALK4/ALK7介导下主要激活Smad2/3,但部分二聚体亦可通过ALK2激活Smad1/5,提示配体组成与受体配对共同决定信号分支选择 [3]。
转录组与分子研究一致显示,ACVR1C在白色与棕色脂肪组织中高度富集,并在脂肪细胞分化晚期显著上调 [4][1]。人类脂肪组织研究进一步证实其表达与肥胖呈负相关,并与糖脂代谢指标密切相关,支持Activin B–ALK7轴在脂肪稳态调控中的作用 [5]。此外,ACVR1C在神经系统与胰岛β细胞中亦有表达,且存在多种剪接变体,不同异构体在信号传导能力上存在差异 [6][1]。例如,Alk7-v3无法有效传递Nodal-Cripto信号,提示转录变体可在不改变受体表达的情况下重塑信号输出。其表达还可受代谢刺激调控,如β3-_肾_上_腺_素_激动剂可下调棕色脂肪组织中的ALK7表达,反映其与交感-代谢通路的交叉调控 [6]。近期转录组学研究还将ACVR1C与神经内分泌发育相联系,显示其通过Smad2/3介导的染色质调控抑制Mkrn3表达,从而影响青春期启动 [7]。
2.2 关键配体识别与受体激活机制
在配体层面,Activin E(INHBE)被认为是ACVR1C的重要功能性配体,可通过该受体激活Smad2/3通路并调控能量代谢相关基因表达 [9][10]。药理学阻断实验显示,小分子抑制剂SB431542可抑制Activin E诱导的Smad2/3磷酸化及下游转录激活,支持其信号经ALK7传导 [10]。胚胎模型研究表明,Nodal信号在早期发育阶段主要依赖ALK4,而ALK7在胚胎发生中并非必需,更可能承担组织特异性或配体特异性功能 [11–13]。
在功能层面,Activin E–ACVR1C轴在代谢调控中的作用呈现表型差异。一方面,小鼠体内研究表明该通路抑制脂解、促进脂肪储存并抑制PPARγ相关基因,可能增加代谢风险 [9];另一方面,体外棕色/米色脂肪细胞研究显示Activin E可通过ALK7/Smad2/3上调Ucp1与Fgf21,提示其在特定谱系中促进产热与能量消耗 [10]。这些差异可由研究模型、细胞类型、剂量与时程以及受体微环境差异解释,强调信号偏倚与染色质背景在决定转录结果中的关键作用。由于缺乏系统的亲和力测定与高分辨率结构数据,配体-受体选择性的分子基础仍待阐明 [11]。
3. ACVR1C/ALK7介导的信号转导通路
ACVR1C/ALK7作为TGF-β超家族信号网络中的关键节点,可将胞外配体刺激转化为多层级的细胞内反应,其信号输出既包括以Smad2/3为核心的经典转录调控轴,也涵盖多种非经典信号分支。不同通路在时间尺度、空间定位及生物学效应上的分工,使ACVR1C的功能呈现显著的情境依赖性。
3.1 Smad2/3依赖的经典信号通路
在经典模型中,配体结合促使II型受体与ACVR1C形成复合体,II型受体对ACVR1C的GS区进行磷酸化,从而激活其激酶活性并招募Smad2/3。被磷酸化的Smad2/3与共Smad4形成复合物转位入核,调控靶基因转录 [14]。该轴在脂肪细胞分化、胰岛β细胞功能以及多种肿瘤细胞命运决定中具有核心作用。
在肿瘤模型中,ACVR1C-Smad2信号被证明可在特定背景下促进肿瘤细胞侵袭与生长。例如,在视网膜母细胞瘤中,ACVR1C表达上调并伴随Smad2活性增强,抑制该通路可显著降低侵袭与存活能力,而Smad3的作用相对有限 [16]。这些结果提示,尽管Smad2与Smad3在结构上高度相似,但在ACVR1C信号输出中可能承担不同的功能权重,其差异性可能由受体-配体组合及核内协同因子决定 [15]。
3.2 非经典信号通路与信号偏倚
除经典Smad通路外,ACVR1C还可通过β-arrestin、Ral GTPase等介导非经典信号。相关研究显示,Nodal-ALK7信号可在胞质中形成稳定复合体,调控受体内吞、ERK1/2激活及细胞骨架重塑,从而快速影响细胞迁移与侵袭行为 [17]。在胰腺癌模型中,ALK7通过β-catenin/MMP轴促进基底膜破坏与肿瘤细胞入血,强调其非Smad分支在转移过程中的作用 [18]。
此外,ACVR1C还可通过抑 PPARγ表达调控巨噬细胞与血管平滑肌细胞表型,促进炎症反应及血管重塑 [19][20]。这些发现表明,ACVR1C的信号输出并非单一路径,而是由多条并行通路共同塑造,其偏向性取决于细胞类型、配体浓度及受体微环境。
4. ACVR1C/ALK7 在疾病发生发展中的作用
4.1 肿瘤发生、进展与转移
在多种实体瘤中,ACVR1C可通过Smad2/3介导的转录程序诱导细胞凋亡并抑制肿瘤扩散,被视为组织内在的稳态屏障 [23][24]。临床样本分析显示,ACVR1C表达降低常与不良预后相关,如胆囊癌、乳腺癌等 [25–28]。
然而,在结直肠癌、视网膜母细胞瘤及胰腺导管腺癌等背景下,ACVR1C又可被肿瘤细胞重新利用,通过自分泌或旁分泌回路促进EMT、干性维持及转移 [29–32]。这些相互矛盾的现象凸显了ACVR1C在肿瘤中的高度情境依赖性,其功能取决于配体谱、信号通路偏向及肿瘤微环境状态。
4.2 代谢性疾病与脂肪组织功能
群体遗传学研究为ACVR1C在人体脂肪分布中的因果作用提供了有力证据。UK Biobank的罕见变异分析显示,ACVR1C功能缺失与更有利的脂肪分布及血脂谱相关 [38]。功能研究进一步表明,ACVR1C通过抑制脂解和调控PPARγ信号影响脂肪储存与胰岛素敏感性 [21]。
需要注意的是,ACVR1C同时在胰岛β细胞中参与调控胰岛素基因表达和细胞存活,过度抑制该通路可能带来胰岛功能风险 [42–44]。因此,其作为代谢疾病治疗靶点的开发需强调组织特异性与安全窗评估。
4.3 心血管、生殖与神经系统疾病
在心血管系统中,ACVR1C在压力超负荷条件下可抑制病理性心肌肥大,而在糖尿病背景下其激活却可能促进心肌凋亡与纤维化 [45–47]。在妊娠相关疾病中,Nodal-ALK7信号抑制滋养层细胞侵袭,被认为与先兆子痫的发生相关 [48],[49]。
在神经系统中,ACVR1C既参与缺血后神经保护,也在突触可塑性和记忆形成中发挥作用 [8],提示其在急性损伤与慢性神经退行性改变中可能具有不同的干预价值。
5. ACVR1C/ALK7靶向药物最近研究进展
ACVR1C/ALK7作为TGF-β超家族中的重要信号受体,在代谢调控、肿瘤进展等多种生理与病理过程中发挥关键作用,近年来已成为新兴的治疗靶点。目前,针对ACVR1C的靶向药物研发主要集中在siRNA、小分子抑制剂和单克隆抗体等不同策略上,适应症涵盖肥胖、肿瘤、糖尿病肾病及新型冠状病毒感染等疾病。部分在研管线整理如下表:
| 药物 | 作用机制 | 药物类型 | 在研适应症 | 在研机构 | 最高研发阶段 |
|---|---|---|---|---|---|
| ARO-ALK7 | ALK7抑制剂 | siRNA | 肥胖 | Arrowhead Pharmaceuticals, Inc. | 临床1/2期 |
| A-83-01 | ALK4抑制剂 | ALK5抑制剂 | ALK7抑制剂 | 化学药 | 肿瘤 | Japanese Foundation for Cancer Research | 临床前 |
| ALN-2232 | ALK7抑制剂 | siRNA | 肥胖 | Alnylam Pharmaceuticals, Inc. | 临床前 |
| SB-431542 | ALK4抑制剂 | ALK5抑制剂 | ALK7抑制剂 | 小分子化药 | 新型冠状病毒感染 | Indian Council of Medical Research | 临床前 |
| ALK7 siRNA (时安生物) | ALK7抑制剂 | siRNA | 肥胖 | 苏州时安生物技术有限公司 | 临床前 |
| ITI-8000 | ALK7抑制剂 | 单克隆抗体 | 肿瘤 | Immunomic Therapeutics, Inc. | 临床前 |
| Protocatechuic aldehyde | ALK7 modulators | 小分子化药 | 糖尿病溃疡 | 上海中医药大学附属曙光医院 | 临床前 |
| SB-505124 | ALK4抑制剂 | ALK5抑制剂 | ALK7抑制剂 | 小分子化药 | - | GSK Plc | 临床前 |
| SGB-ALK7 | ALK7抑制剂 | siRNA | 肥胖 | 苏州圣因生物医药有限公司 | 临床前 |
(数据截止到2025年12月26日,来源于synapse)
6. ACVR1C/ALK7相关研究工具
ACVR1C/ALK7是连接发育生物学、代谢调控与疾病病理的重要信号节点。其生物学效应高度依赖细胞类型、配体环境及信号偏倚,这既赋予其治疗潜力,也增加了转化难度。华美生物提供ACVR1C/ALK7抗体产品,助力您进行相关机制研究及靶向药物开发。
● ACVR1C/ALK7抗体
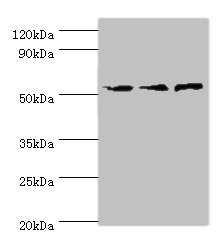
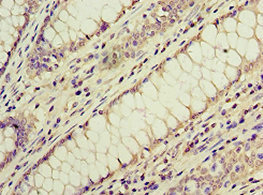
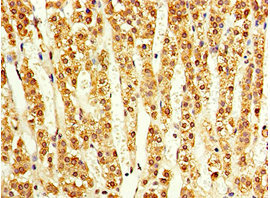
ACVR1C Antibody; CSB-PA854112ESR1HU
[1] Masahiro Kogame, Shinji Matsuo, Masashi Nakatani, Akira Kurisaki, Hiromu Nishitani, Kunihiro Tsuchida, Hiromu Sugino.(2006). ALK7 is a novel marker for adipocyte differentiation.
[2] Luisa Calvanese, Annamaria Sandomenico, Andrea Caporale, Annalia Focà, Giuseppina Focà, Gabriella D'Auria, Lucia Falcigno, Menotti Ruvo.(2015). Conformational features and binding affinities to Cripto, ALK7 and ALK4 of Nodal synthetic fragments.
[3] Oddrun Elise Olsen, Hanne Hella, Samah Elsaadi, Carsten Jacobi, Erik Martı́nez-Hackert, Toril Holien.(2020). Activins as Dual Specificity TGF-β Family Molecules: SMAD-Activation via Activin- and BMP-Type 1 Receptors.
[4] Yan Song, Jinsoo Ahn, Yeunsu Suh, Michael E Davis, Kichoon Lee.(2013). Identification of novel tissue-specific genes by analysis of microarray databases: a human and mouse model.
[5] Lena M S Carlsson, Peter Jacobson, Andrew Walley, Philippe Froguel, Lars Sjöström, Per-Arne Svensson, Kajsa Sjöholm.(2009). ALK7 expression is specific for adipose tissue, reduced in obesity and correlates to factors implicated in metabolic disease.
[6] Masaru Murakami, Mitsuyuki Shirai, Ryo Ooishi, Asako Tsuburaya, Kumiko Asai, Osamu Hashimoto, Kenji Ogawa, Yoshii Nishino, Masayuki Funaba.(2013). Expression of activin receptor-like kinase 7 in adipose tissues.
[7] Dor Shalev, G. Golan, Lilach Pnueli, Anat Kahan, Yael Mandel‐Gutfreund, Philippa Melamed.(2025). High-throughput transcriptome analysis reveals a developmental increase in Acvr1c which mediates epigenetic repression of the gene encoding the pubertal brake, Makorin ring finger protein 3.
[8] Meilian Liu, Yudie Li, Song Han, Hongyu Wang, Junfa Li.(2022). Activin A alleviates neuronal injury through inhibiting cGAS-STING-mediated autophagy in mice with ischemic stroke.
[9] Rene C. Adam, Dwaine S. Pryce, Joseph S. Lee, Yuanqi Zhao, Ivory J. Mintah, Soo Min, Gábor Halász, Jason Mastaitis, Gurinder S. Atwal, Senem Aykul, Vincent Idone, Aris N. Economides, Luca A. Lotta, Andrew Murphy, George D. Yancopoulos, Mark W. Sleeman, Viktoria Gusarova.(2023). Activin E–ACVR1C cross talk controls energy storage via suppression of adipose lipolysis in mice.
[10] Maho Sakaki, Yuji Kamatari, Akira Kurisaki, Masayuki Funaba, Osamu Hashimoto.(2024). Activin E upregulates uncoupling protein 1 and fibroblast growth factor 21 in brown adipocytes.
[11] .(2021). Decision letter: Regulation of Nodal signaling propagation by receptor interactions and positive feedback.
[12] Hannes Preiß, Anna C. Kögler, David Mörsdorf, Daniel Čapek, Gary H. Soh, Katherine W. Rogers, Hernán Morales‐Navarrete, María Almuedo‐Castillo, Patrick Müller.(2022). Author response: Regulation of Nodal signaling propagation by receptor interactions and positive feedback.
[13] Henrik Jörnvall, Eva Reissmann, Olov Andersson, Mehrnaz Mehrkash, Carlos F Ibáñez.(2004). ALK7, a receptor for nodal, is dispensable for embryogenesis and left-right patterning in the mouse.
[14] Lilianna Solnica‐Krezel.(2021). Editor's evaluation: Regulation of Nodal signaling propagation by receptor interactions and positive feedback.
[15] G Fu, C Peng.(2011). Nodal enhances the activity of FoxO3a and its synergistic interaction with Smads to regulate cyclin G2 transcription in ovarian cancer cells.
[16] Laura Asnaghi, David T White, Nolan Key, Joshua Choi, Alka Mahale, Hind Alkatan, Deepak P Edward, Sahar M Elkhamary, Saleh Al-Mesfer, Azza Maktabi, Christopher G Hurtado, Grace Y Lee, Angel M Carcaboso, Jeff S Mumm, Leen Abu Safieh, Charles G Eberhart.(2019). ACVR1C/SMAD2 signaling promotes invasion and growth in retinoblastoma.
[17] Jeffrey Law, Guihua Zhang, Magdalena Dragan, Lynne-Marie Postovit, Moshmi Bhattacharya.(2014). Nodal signals via β-arrestins and RalGTPases to regulate trophoblast invasion.
[18] Anna M Kolarzyk, Yujin Kwon, Elizabeth Oh, Keng-Jung Lee, Su-Yeon Cho, Issahy Cano, Renhao Lu, Tae Joon Kwak, Jaehyun Lee, Gigi Wong, Andrew H Kim, Omar Gandarilla, Manuel Hidalgo, Won Kyu Kim, Esak Lee.(2025). Non-canonical ALK7 pathways promote pancreatic cancer metastasis through β-catenin/MMP-mediated basement membrane breakdown and intravasation.
[19] Wen-Lin Cheng, Quan Zhang, Jian-Lei Cao, Xi-Lu Chen, Wenyan Li, Lin Zhang, Sheng-Ping Chao, Fang Zhao.(2021). ALK7 Acts as a Positive Regulator of Macrophage Activation through Down-Regulation of PPARγ Expression.
[20] Fu-Han Gong, Wen-Lin Cheng, Quan Zhang, Xi-Lu Chen, Jian-Lei Cao, Ting Yang, Wen-Hao Song, Fang Zhao.(2020). ALK7 Promotes Vascular Smooth Muscle Cells Phenotypic Modulation by Negative Regulating PPARγ Expression.
[21] Satomi Yogosawa, Shin Mizutani, Yoshihiro Ogawa, Tetsuro Izumi.(2013). Activin receptor-like kinase 7 suppresses lipolysis to accumulate fat in obesity through downregulation of peroxisome proliferator-activated receptor γ and C/EBPα.
[22] Yu-Dan Tian, Min Hwa Chung, Qing-Ling Quan, Dong Hun Lee, Eun Ju Kim, Jin Ho Chung.(2021). UV-Induced Reduction of ACVR1C Decreases SREBP1 and ACC Expression by the Suppression of SMAD2 Phosphorylation in Normal Human Epidermal Keratinocytes.
[23] Guoxiong Xu, Hong Zhou, Qinghua Wang, Nelly Auersperg, Chun Peng.(2006). Activin receptor-like kinase 7 induces apoptosis through up-regulation of Bax and down-regulation of Xiap in normal and malignant ovarian epithelial cell lines.
[24] Iacovos P Michael, Sadegh Saghafinia, Mélanie Tichet, Nadine Zangger, Ilaria Marinoni, Aurel Perren, Douglas Hanahan.(2019). ALK7 Signaling Manifests a Homeostatic Tissue Barrier That Is Abrogated during Tumorigenesis and Metastasis.
[25] J Li, Z Yang, Q Zou, Y Yuan, J Li, L Liang, G Zeng, S Chen.(2014). PKM2 and ACVR 1C are prognostic markers for poor prognosis of gallbladder cancer.
[26] Fancai Zeng, Guoxiong Xu, Tiejun Zhou, Chengwan Yang, Xinyan Wang, Chun Peng, Hong Zhou.(2012). Reduced expression of activin receptor-like kinase 7 in breast cancer is associated with tumor progression.
[27] Tingting Hu, Fengxi Su, Wenguo Jiang, D Alwyn Dart.(2017). Overexpression of Activin Receptor-like Kinase 7 in Breast Cancer Cells Is Associated with Decreased Cell Growth and Adhesion.
[28] Shahan Mamoor.(2021). Differential expression of activin A receptor type 1C in cancers of the breast.
[29] Huaixiang Zhou, Qunlong Jin, Fu Zhang, Yuan‐Han Yang, Yunfei Gao, Niu Wang, Bo Zhao, Long Gui, Li Jiang, Zijing Zhu, Ying Zhang, Yulong He, Ying Zhang, Shouqing Luo, Fu Li, Xudong Wu, Junjing Zhang, Xuetong Shen, Tao Wang, Youheng Jiang, Ningning Li.(2025). A Paracrine-to-Autocrine Shunt of GREM1 Fuels Colorectal Cancer Metastasis via ACVR1C.
[30] Vimalan Rengganaten, Chiu-Jung Huang, Mong‐Lien Wang, Yueh Chien, Ping‐Hsing Tsai, Yuan‐Tzu Lan, Hooi Tin Ong, Shih‐Hwa Chiou, Kong‐Bung Choo.(2023). Circular RNA ZNF800 (hsa_circ_0082096) regulates cancer stem cell properties and tumor growth in colorectal cancer.
[31] Laura Asnaghi, David T White, Lynn Yoon, Antoinette Price, Grace Y Lee, Arpan Sahoo, Jeff S Mumm, Charles G Eberhart.(2019). Downregulation of Nodal inhibits metastatic progression in retinoblastoma.
[32] Duc-Huy T Nguyen, Esak Lee, Styliani Alimperti, Robert J Norgard, Alec Wong, Jake June-Koo Lee, Jeroen Eyckmans, Ben Z Stanger, Christopher S Chen.(2019). A biomimetic pancreatic cancer on-chip reveals endothelial ablation via ALK7 signaling.
[33] Chunming Zhang, Wenjing Hao, Xinfang Wang, Huina Guo, Long He, Yang Jiao, Ying Wang, Xiwang Zheng, Zhongxun Li, Qi Han, Liqi Wen, Hongliang Liu.(2025). MEIS1-regulated miR-488-3p suppresses the malignant progression of laryngeal squamous cell carcinoma by targeting ACVR1C.
[34] Elham Kashani, Désirée Schnidrig, Ali Hashemi Gheinani, Martina Selina Ninck, Philipp Zens, Theoni Maragkou, Ulrich Baumgartner, Philippe Schucht, Gunnar Rätsch, Mark A. Rubin, Andrej Benjak, Rémy Bruggmann, Federico Comoglio, André Kahles, Irene Keller, Charlotte K.Y. Ng, Salvatore Piscuoglio, Laurie Prélot, Gunnar Rätsch, Mark A. Rubin, Désirée Schnidrig, Senija Selimovic-Hamza, Tinu M. Thomas, Sabina Berezowska, Charlotte K.Y. Ng, Erik Vassella.(2022). Integrated longitudinal analysis of adult grade 4 diffuse gliomas with long-term relapse interval revealed upregulation of TGF-β signaling in recurrent tumors.
[35] Zhixiao Xu, Chengshui Chen.(2021). Abnormal Expression and Prognostic Significance of Bone Morphogenetic Proteins and Their Receptors in Lung Adenocarcinoma.
[36] Si-Yun Lin, Hou Huang, Jinjie Yu, Feng Su, Tian Jiang, Shaoyuan Zhang, Lu Lv, Tao Long, Huiwen Pan, Jun-Qing Qi, Qiang Zhou, Weifeng Tang, Guowen Ding, Liming Wang, Lijie Tan, Jun Yin.(2024). Activin A receptor type 1C single nucleotide polymorphisms associated with esophageal squamous cell carcinoma risk in Chinese population.
[37] Shuang Zheng, Caizheng Wang, Junhui Fu, Jinfan Shao.(2025). Investigating Overlapping Immune-related Genetic Markers in Cholangiocarcinoma and Inflammatory Bowel Disease for Predictive Prognosis.
[38] Mine Koprulu, Yajie Zhao, Eleanor Wheeler, Liang Dong, Nuno Rocha, Chen Li, John D Griffin, Satish Patel, Marcel Van de Streek, Craig A. Glastonbury, Isobel D. Stewart, Felix R. Day, Jian’an Luan, Nicholas Bowker, Laura B. L. Wittemans, Nicola D. Kerrison, Lina Cai, Debora Lucarelli, Inês Barroso, Mark I. McCarthy, Robert A. Scott, Vladimı́r Saudek, Kerrin S. Small, Nicholas J. Wareham, Robert K. Semple, John R. B. Perry, Stephen O’Rahilly, Luca A. Lotta, Claudia Langenberg, David B. Savage.(2021). Identification of Rare Loss-of-Function Genetic Variation Regulating Body Fat Distribution.
[39] Wenchao Zhang, Hui Wang, Wei Zhang, Ruijuan Lv, Zhihao Wang, Yuanyuan Shang, Yun Zhang, Ming Zhong, Yuguo Chen, Mengxiong Tang.(2013). ALK7 gene polymorphism is associated with metabolic syndrome risk and cardiovascular remodeling.
[40] Katherine A Kentistou, Brandon E M Lim, Lena R Kaisinger, Valgerdur Steinthorsdottir, Luke N Sharp, Kashyap A Patel, Vinicius Tragante, Gareth Hawkes, Eugene J Gardner, Thorhildur Olafsdottir, Andrew R Wood, Yajie Zhao, Gudmar Thorleifsson, Felix R Day, Susan E Ozanne, Andrew T Hattersley, Stephen O'Rahilly, Kari Stefansson, Ken K Ong, Robin N Beaumont, John R B Perry, Rachel M Freathy.(2024). Rare variant associations with birth weight identify genes involved in adipose tissue regulation, placental function and insulin-like growth factor signalling.
[41] Pawanrat Tangseefa, Hong Jin, Houyu Zhang, Meng Xie, Carlos F. Ibáñez.(2024). Human ACVR1C missense variants that correlate with altered body fat distribution produce metabolic alterations of graded severity in knock-in mutant mice.
[42] Rie Watanabe, Zhen-Ping Shen, Kinsuke Tsuda, Yuichiro Yamada.(2008). Insulin gene is a target in activin receptor-like kinase 7 signaling pathway in pancreatic beta-cells.
[43] Fang Zhao, Fengjie Huang, Mengxiong Tang, Xiaoming Li, Nina Zhang, Akis Amfilochiadis, Yiming Li, Renming Hu, Tianru Jin, Chun Peng, Qinghua Wang.(2012). Nodal induces apoptosis through activation of the ALK7 signaling pathway in pancreatic INS-1 β-cells.
[44] Junfeng Li, Zhihong Wang, Liwei Ren, Linling Fan, Wenjuan Liu, Yaojing Jiang, Harry K Lau, Rui Liu, Qinghua Wang.(2018). Antagonistic interaction between Nodal and insulin modulates pancreatic β-cell proliferation and survival.
[45] He Huang, Yanhong Tang, Gang Wu, Yang Mei, Wanli Liu, Xiaoxiong Liu, Nian Wan, Yu Liu, Congxin Huang.(2015). ALK7 protects against pathological cardiac hypertrophy in mice.
[46] Lin Liu, Xin Zhou, Qiyu Zhang, Li Li, Yuanyuan Shang, Zhihao Wang, Ming Zhong, Yuguo Chen, Wei Zhang, Mengxiong Tang.(2022). Activin receptor-like kinase 7 silencing alleviates cardiomyocyte apoptosis, cardiac fibrosis, and dysfunction in diabetic rats.
[47] Wen-bo Li, Jing Zhao, Lin Liu, Zhi-hao Wang, Lu Han, Ming Zhong, Yun Zhang, Wei Zhang, Meng-xiong Tang.(2015). Silencing of activin receptor-like kinase 7 alleviates aortic stiffness in type 2 diabetic rats.
[48] Lubna Nadeem, Sadia Munir, Guodong Fu, Caroline Dunk, Dora Baczyk, Isabella Caniggia, Stephen Lye, Chun Peng.(2011). Nodal signals through activin receptor-like kinase 7 to inhibit trophoblast migration and invasion: implication in the pathogenesis of preeclampsia.
[49] Mengsi Hu, Yao Wang, Yanping Meng, Jinxiu Hu, Jiao Qiao, Junhui Zhen, Decai Liang, Minghua Fan.(2022). Hypoxia induced-disruption of lncRNA TUG1/PRC2 interaction impairs human trophoblast invasion through epigenetically activating Nodal/ALK7 signalling.



