武汉华美生物工程有限公司CUSABIO®品牌商
15 年
手机商铺
- NaN
- 0
- 0
- 2
- 2
推荐产品
公司新闻/正文
代谢枢纽,重编程疾病:SLC3A2——从氨基酸转运机制到新兴治疗靶点
189 人阅读发布时间:2025-11-07 10:14
SLC3A2(CD98hc)远非普通的转运蛋白,而是连接氨基酸代谢与细胞信号的核心枢纽。它在癌症、神经、代谢及免疫疾病中扮演关键角色,正成为极具潜力的新兴治疗靶点。本文将沿此脉络,阐释其从氨基酸转运机制到新兴治疗靶点的完整路径。
1. SLC3A2为何成为代谢与疾病的核心枢纽?
2. SLC3A2如何执行其关键的生物学功能?
3. SLC3A2如何调控关键的细胞信号通路?
4. SLC3A2 相关疾病
5. 靶向SLC3A2药物的研究进展如何?
6. SLC3A2研究工具
1. SLC3A2为何成为代谢与疾病的核心枢纽?
SLC3A2(溶质载体家族3成员2,亦称4F2hc或CD98hc)是一种II型跨膜糖蛋白,隶属于SLC3家族。其主要功能是与SLC7家族轻链亚基形成异二聚体氨基酸转运体(HATs),共同介导氨基酸跨膜转运并调控细胞代谢与信号活动 [1,2]。
SLC3A2由短N端胞质区、单跨膜螺旋(TM)和较大的C端胞外域(ED)组成。胞外域虽然结构上类似细菌葡萄糖苷酶,但无催化活性,主要作用于蛋白相互作用与构象稳定 [1]。通过保守二硫键,SLC3A2与SLC7家族成员(如LAT1、xCT等)形成复合物以实现其转运功能。在此异二聚体中,SLC3A2负责将轻链亚基运送至细胞膜并维持其稳定性,而底物识别与离子选择性由轻链亚基决定 [2,3]。
SLC3A2在多种高代谢组织中高表达,包括血脑屏障(BBB)、胎盘及免疫活化细胞 [4-7]。胚胎发育中其缺失会导致致死,而在肿瘤细胞中,SLC3A2通常呈显著上调状态,以增强氨基酸供给、促进生长与代谢重编程 [8,9]。这些特征使SLC3A2成为连接营养代谢与信号调控的重要枢纽。
2. SLC3A2如何执行其关键的生物学功能?
2.1 氨基酸转运机制
SLC3A2通过与不同轻链亚基形成复合物介导多类型氨基酸转运:
- 4F2hc-LAT1(system L):钠非依赖性转运大中性氨基酸(如亮氨酸、异亮氨酸),遵循“交替访问模型”完成跨膜交换,同时可转运L-DOPA、甲状腺激素等底物 [9,10]。
- 4F2hc-xCT(system xc?):介导胞外胱氨酸与胞内谷氨酸的1:1交换,为谷胱甘肽(GSH)合成提供半胱氨酸,维持细胞氧化还原平衡 [9]。
- 4F2hc-LAT2及4F2hc-y+LAT1/2分别参与小分子氨基酸重吸收及阳离子氨基酸代谢,在肾脏、肠道及免疫细胞中发挥重要作用 [11,12]。
2.2 N-糖基化与定位调控
N-糖基化是SLC3A2功能维持的关键修饰。胰腺癌中,Asn365糖基化由B3GNT3催化,显著增强蛋白稳定性与xCT结合,从而维持system xc?活性并抵御铁死亡(ferroptosis)[9]。糖基化缺陷则加速降解并增加铁死亡敏感性。
SLC3A2主要定位于细胞膜,但也可因信号刺激或蛋白互作(如LAPTM4b或DRAM-1)定位至溶酶体膜,参与氨基酸储存与mTORC1信号调控 [10,15]。
3. SLC3A2如何调控关键的细胞信号通路?
3.1 mTORC1信号通路
SLC3A2介导的亮氨酸摄取是mTORC1信号通路激活的关键步骤。亮氨酸通过Sestrin–GATOR及LRS通路调节Rag GTPases活性,促进mTORC1在溶酶体膜上激活,从而促进蛋白质合成与细胞生长 [16,17]。在头颈部鳞癌中,SLC3A2缺失会降低mTORC1活性并触发自噬,而自噬抑制可增强放疗敏感性 [3]。
3.2 整合应激反应(ISR)
当SLC3A2功能受损时,氨基酸减少导致未带电tRNA积累,激活GCN2–eIF2α–ATF4通路。ATF4反向促进SLC3A2及LAT1表达,恢复氨基酸稳态 [19]。这一机制在前列腺癌等高代谢肿瘤中尤为显著。
3.3 Integrin 信号通路
SLC3A2胞内结构域可与整合素β亚基相互作用,影响细胞黏附与迁移 [2,20]。在肝癌中,SLC3A2可抑制β1整合素活化以限制侵袭;而在肾癌中则促进整合素信号,增强迁移与基质黏附能力 [20]。
4. SLC3A2相关疾病
SLC3A2作为氨基酸代谢与信号通路的交叉节点,与多种疾病的发生和进展密切相关。
4.1 癌症
4.1.1前列腺癌
SLC3A2是雄激素受体剪接变体AR-V7的下游靶基因,AR-V7可驱动其表达并通过LAT1复合物激活mTORC1信号,从而支持去势抵抗性前列腺癌(CRPC)生长 [8,11]。临床数据显示,SLC3A2高表达与病理分级升高及不良预后密切相关 [21]。
4.1.2 胰腺癌
在胰腺导管腺癌(PDAC)中,B3GNT3介导的N-糖基化增强SLC3A2稳定性并促进xCT结合,维持system xc?功能与抗氧化能力 [9]。该机制使PDAC细胞对铁死亡具有显著耐受性。临床样本分析显示,SLC3A2和B3GNT3双高表达与生存期缩短密切相关,可作为预后生物标志物。
4.1.3 头颈部鳞状细胞癌(HNSCC)
SLC3A2高表达促进氨基酸转运和DNA修复能力,使肿瘤细胞表现出放疗抗性 [3]。SLC3A2敲除或mTORC1抑制可削弱放射抵抗,而联合自噬抑制进一步增强治疗效果,提示其为潜在放疗敏感化靶点。
4.1.4 泌尿系统肿瘤
膀胱癌与肾癌中,SLC3A2–LAT1复合物介导亮氨酸吸收,促进细胞增殖和侵袭 [21,22]。SLC3A2高表达与高分级、转移风险和预后不良密切相关,提示其在泌尿系统肿瘤中具有潜在诊断与治疗价值。
4.1.5 其他恶性肿瘤
在肝细胞癌、乳腺癌及脑胶质瘤中,SLC3A2同样被发现调控mTORC1通路及整合素信号通路,从而影响细胞代谢、迁移及对抗应激能力。这些结果支持其作为“代谢–信号”双靶点在精准肿瘤治疗中的潜力。
4.2 神经系统疾病
SLC3A2在血脑屏障(BBB)中高表达,与脑内氨基酸稳态维持密切相关。LAT1基因突变(Ala246Val、Pro375Leu)会损害SLC3A2-LAT1复合物功能,导致脑内氨基酸失衡、激活ISR通路并产生自闭症谱系障碍(ASD)相关表型 [22,23]。此外,在帕金森病中,SLC3A2–LAT1复合物负责L-DOPA的脑转运,支链氨基酸(BCAAs)可竞争结合,降低L-DOPA治疗效率,解释了部分患者的药物耐受现象 [24]。
4.3 代谢性疾病
SLC3A2与BCAAs代谢、胰岛素抵抗及2型糖尿病(T2DM)密切相关。SLC3A2-LAT1复合物介导的BCAAs摄取可过度激活mTORC1,从而抑制胰岛素信号并诱导胰岛素抵抗 [25]。此外,高血糖可抑制AMPK信号并下调SLC3A2表达,进一步升高血浆BCAAs水平,形成恶性代谢循环 [25]。在胰岛β细胞中,SLC3A2参与氨基酸摄取与胰岛素合成,其功能障碍可导致分泌不足并加重代谢失衡 [26]。
4.4 炎症与免疫相关疾病
SLC3A2在免疫细胞代谢重编程中具有关键作用。
- 在T细胞活化过程中,其表达显著上调,通过增强氨基酸供应支持效应T细胞分化与细胞因子分泌。抑制SLC3A2可减弱T细胞活化并缓解Th2介导的过敏性炎症 [27]。
- 在B细胞中,SLC3A2介导亮氨酸摄取激活mTORC1通路,促进炎症因子(IL-6、TNF-α)产生,与类风湿关节炎病情活动度呈正相关 [28]。
这些结果表明,SLC3A2不仅是代谢通道蛋白,也参与免疫信号的代谢调控,是炎症性疾病治疗的新潜在靶点。
5. 靶向SLC3A2药物的研究进展如何?
SLC3A2已成为新兴的抗癌药物靶点。目前的研究方向涵盖单克隆抗体、小分子抑制剂及抗体药物偶联物(ADC)等类型,主要集中于血液肿瘤、实体瘤和脑癌等领域,均处于临床前阶段。
| 药物 | 作用机制 | 药物类型 | 在研适应症(疾病名) | 最高研发阶段 |
|---|---|---|---|---|
| MAb52-4.2 | SLC3A2调节剂 | 单克隆抗体 | 血液肿瘤 | 实体瘤 | 临床前 |
| 211At-TLX-102 | SLC3A2抑制剂 | SLC7A5抑制剂 | 小分子化药 | 治疗用放射药物 | 脑癌 | 临床前 |
| c-SF-25 Mab(Kagoshima University) | SLC3A2抑制剂 | 单克隆抗体 | 成人T细胞白血病/淋巴瘤 | 临床前 |
| NPB15 | SLC3A2抑制剂 | 单克隆抗体 | 肝细胞癌 | 临床前 |
| HH018-sesutecan | SLC3A2抑制剂 | ADC | 肿瘤 | 临床前 |
(数据截止到2025年10月30日,来源于synapse数据库)
6. SLC3A2研究工具
● SLC3A2重组蛋白
Recombinant Human Amino acid transporter heavy chain SLC3A2 (SLC3A2), partial (Active); CSB-MP021640HU1
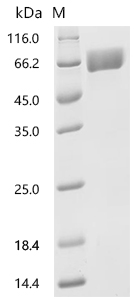
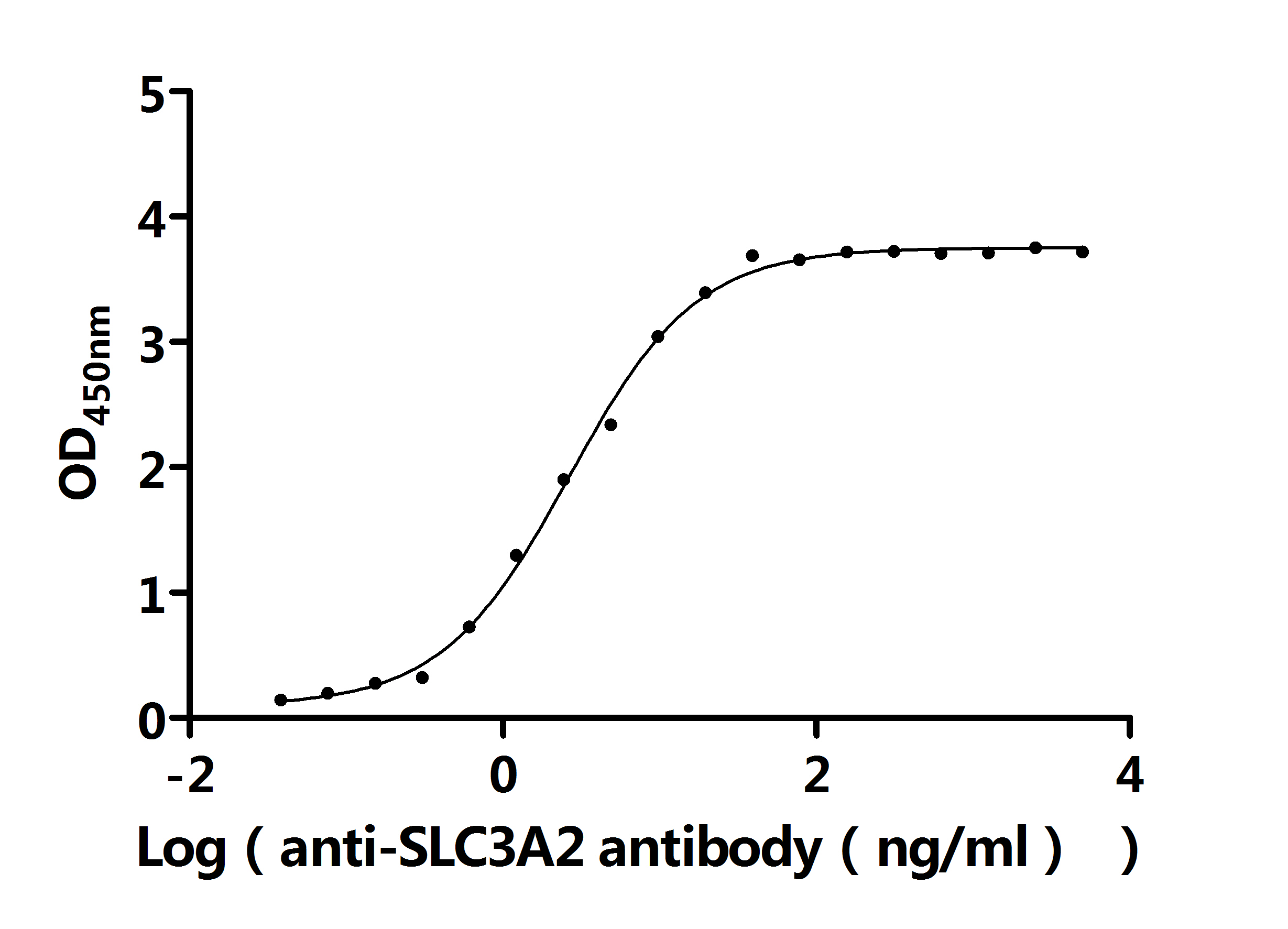
● SLC3A2抗体
SLC3A2 Recombinant Monoclonal Antibody
CSB-RA021640MA1HU
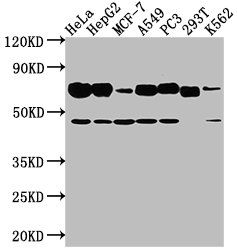
SLC3A2 Antibody
CSB-PA021640LA01HU
● SLC3A2细胞株
HEK293T/Human SLC7A11 & SLC3A2 Stable Cell Line; CSB-SC5338HU
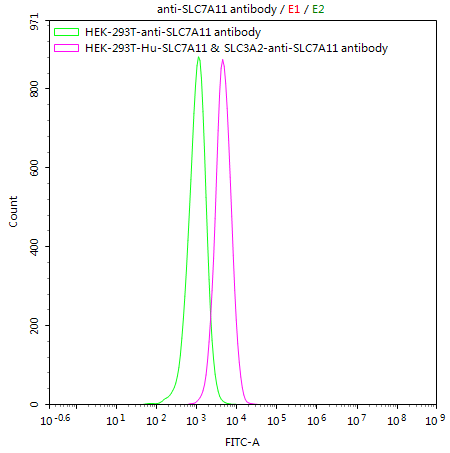
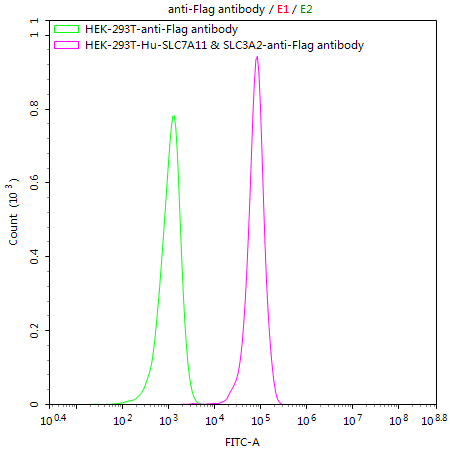
参考文献:
[1] Sub-Nanometer Cryo-EM Density Map of the Human Heterodimeric Amino Acid Transporter 4F2hc-LAT2.
[2] Kahlhofer J, Teis D. The human LAT1–4F2hc (SLC7A5–SLC3A2) transporter complex: Physiological and pathophysiological implications[J]. Basic Clin Pharmacol Toxicol, 2023, 133(5):459-472.
[3] Digomann D, Linge A, Dubrovska A. SLC3A2/CD98hc, autophagy and tumor radioresistance: a link confirmed[J]. Autophagy, 2019, 15(10):1850-1851.
[4] Boado RJ, Li JY, Nagaya M, et al. Selective expression of the large neutral amino acid transporter at the blood–brain barrier[J]. Proc Natl Acad Sci USA, 1999, 96(21):12079-12084.
[5] Matsuo H, Tsukada S, Nakata T, et al. Expression of a system L neutral amino acid transporter at the blood–brain barrier[J]. Neuroreport, 2000, 11(16):3507-3511.
[6] Laudicella R, Albano D, Alongi P, et al. (18)F-Facbc in Prostate Cancer: A Systematic Review and Meta-Analysis[J]. Cancers, 2019, 11(1348).
[7] Nii T, Segawa H, Taketani Y, et al. Molecular events involved in up-regulating human Na+-independent neutral amino acid transporter LAT1 during T-cell activation[J]. Biochem J, 2001, 358(Pt 3):693-704.
[8] Zhao X, Sakamoto S, Maimaiti M, et al. Contribution of LAT1-4F2hc in Urological Cancers via Toll-like Receptor and Other Vital Pathways[J]. Cancers, 2022, 14(1):229.
[9] Ma H, Chen X, Mo S, et al. Targeting N-glycosylation of 4F2hc mediated by glycosyltransferase B3GNT3 sensitizes ferroptosis of pancreatic ductal adenocarcinoma[J]. Cell Death Differ, 2023, 30(1988-2004).
[10] Helgudóttir S-S, Johnsen K-B, Routhe L-G, et al. Upregulation of Transferrin Receptor 1 (TfR1) but Not Glucose Transporter 1 (GLUT1) or CD98hc at the Blood–Brain Barrier in Response to Valproic Acid[J]. Cells, 2024, 13(14):1181.
[11] Sugiura M, Sato H, Okabe A, et al. Identification of AR-V7 downstream genes commonly targeted by AR/AR-V7 and specifically targeted by AR-V7 in castration resistant prostate cancer[J]. Transl Oncol, 2021, 14(10):100915.
[12] Torrents D, Estévez R, Pineda M, et al. Identification and characterization of a membrane protein (y+L amino acid transporter-1) that associates with 4F2hc to encode the amino acid transport activity y+L[J]. J Biol Chem, 1998, 273(49):32437-32445.
[13] Lee Y, Wiriyasermkul P, Jin C, et al. Cryo-EM structure of the human L-type amino acid transporter 1 in complex with glycoprotein CD98hc[J]. Nat Struct Mol Biol, 2019, 26(6):510-517.
[14] Deuschle F-C, Schiefner A, Brandt C, et al. Design of a surrogate Anticalin protein directed against CD98hc for preclinical studies in mice[J]. Protein Sci, 2020, 29(1774-1783).
[15] Brown EJ, Albers MW, Shin TB, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex[J]. Nature, 1994, 369(6483):756-758.
[16] Saxton RA, Knockenhauer KE, Wolfson RL, et al. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway[J]. Science, 2016, 351(6268):53-58.
[17] Han JM, Jeong SJ, Park MC, et al. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway[J]. Cell, 2012, 149(2):410-424.
[18] Park Y, Reyna-Neyra A, Philippe L, et al. mTORC1 balances cellular amino acid supply with demand for protein synthesis through post-transcriptional control of ATF4[J]. Cell Rep, 2017, 19(6):1083-1090.
[19] Cordova RA, Misra J, Amin PH, et al. GCN2 eIF2 kinase promotes prostate cancer by maintaining amino acid homeostasis[J]. Elife, 2022, 11:e81083.
[20] Poettler M, Unseld M, Braemswig K, et al. CD98hc (SLC3A2) drives integrin-dependent renal cancer cell behavior[J]. Mol Cancer, 2013, 12(1):169.
[21] Betsunoh H, Fukuda T, Anzai N, et al. Increased expression of system large amino acid transporter (LAT)-1 mRNA is associated with invasive potential and unfavorable prognosis of human clear cell renal cell carcinoma[J]. BMC Cancer, 2013, 13(509).
[22]Tarlungeanu DC, Deliu E, Dotter CP, et al. Impaired amino acid transport at the blood brain barrier is a cause of autism spectrum disorder[J]. Cell, 2016, 167(6):1481-1494.
[23] Errasti-Murugarren E, Palacín M. Heteromeric amino acid transporters in brain: from physiology to pathology[J]. Neurochem Res, 2022, 47(1):23-36.
[24] Beckers M, Bloem BR, Verbeek MM. Mechanisms of peripheral levodopa resistance in Parkinson’s disease[J]. NPJ Parkinsons Dis, 2022, 8(1):56.
[25] Yamamoto Y, Sawa R, Wake I, et al. Glucose-mediated inactivation of AMP-activated protein kinase reduces the levels of L-type amino acid transporter 1 mRNA in C2C12 cells[J]. Nutr Res, 2017, 47(13-20).
[26] Kobayashi N, Okazaki S, Sampetrean O, et al. CD44 variant inhibits insulin secretion in pancreatic β cells by attenuating LAT1-mediated amino acid uptake[J]. Sci Rep, 2018, 8(1):2785.
[27] Hayashi K, Kaminuma O, Nishimura T, et al. LAT1-specific inhibitor is effective against T cell-mediated allergic skin inflammation[J]. Allergy, 2020, 75(2):463-467.
[28] Torigoe M, Maeshima K, Ozaki T, et al. L-leucine influx through Slc7a5 regulates inflammatory responses of human B cells via mammalian target of rapamycin complex 1 signaling[J]. Mod Rheumatol, 2019, 29(5):885-891.



