武汉华美生物工程有限公司CUSABIO®品牌商
15 年
手机商铺
- NaN
- 0
- 0
- 2
- 2
推荐产品
公司新闻/正文
乙酰胆碱受体α1亚基(CHRNA1):结构、功能机制及相关疾病研究进展
260 人阅读发布时间:2025-10-27 09:45
1. CHRNA1的背景介绍
1.1 CHRNA1的分子结构与功能特性
CHRNA1基因位于人类2号染色体q31.1,编码yanjian型乙酰胆碱受体(nAChR)的α1亚基。该蛋白含457个氨基酸,具备四个跨膜结构域(M1–M4),其中M2形成离子通道孔道,负责阳离子通透 [1]。在神经肌肉接头(NMJ),CHRNA1与β1、δ、ε/γ亚基组装为五聚体受体,介导乙酰胆碱(ACh)信号,引发肌细胞膜去极化与肌肉收缩 [2][3]。
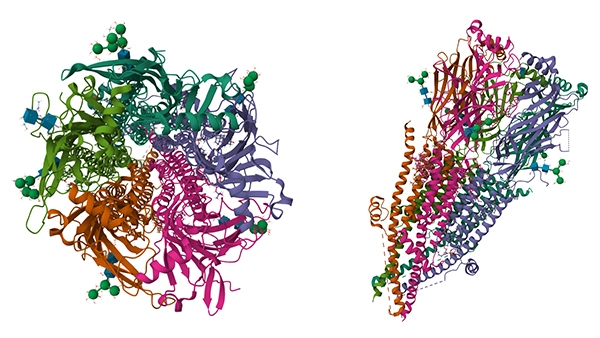
Human muscle nAChR apo state (图源:PDB)
基因敲除实验表明,CHRNA1缺失会导致NMJ突触后膜发育异常并阻断信号传递,突显其在突触形成和功能维持中的关键作用 [2]。此外,CHRNA1胞内环区可与rapsyn结合,确保受体在突触后膜高密度聚集 [4]。其M3-M4连接区的磷酸化修饰还可调控受体内化速率,对突触可塑性具有重要意义 [3]。成人骨骼肌中,ε亚基替代胎儿γ亚基,提高通道电导率,该亚基转换依赖agrin-MuSK信号通路调控 [5]。
1.2 CHRNA1的表达分布
CHRNA1在外周神经系统NMJ终板区高表达,并与乙酰胆碱酯酶共定位,形成高效的突触后致密区 [6][7]。在中枢神经系统(如海马、皮层、脑干),其表达较低,但提示可能参与认知调节和自主神经功能 [8]。
胸腺髓质上皮细胞中CHRNA1的“异位表达”受AIRE调控,与自身免疫耐受及重症肌无力相关 [6]。单细胞测序显示,CHRNA1在脊髓前角运动神经元中呈阶段性高峰,与NMJ发育同步 [9]。此外,在肺动脉平滑肌细胞中也可检测到CHRNA1转录本,其水平与线粒体复合物Ⅰ活性呈负相关,可能参与血管张力调控 [10]。
1.3 CHRNA1与相关受体的相互作用
CHRNA1作为nAChR的核心亚基,其N端胞外区含有ACh结合位点,M2跨膜螺旋形成离子通道 [5]。研究表明,CHRNA1与β1的相互作用可影响通道开放概率;当β1突变时,电流幅度下降约60% [11]。胚胎期CHRNA1-γ受体被ε亚基替代后,通道动力学特性明显改变 [1]。
CHRNA1还能与α7或α3β4等亚基形成异源受体,改变钙离子通透性及脱敏速率 [12]。此外,CHRNA1可与5-HT3A、GABAA等受体存在交叉调控,影响电流衰减和受体敏感性 [11][2]。这些互作在病理条件下可能受损,如慢阻肺患者中CHRNA1与α9共定位消失并伴随炎症因子异常升高 [11]。
2. CHRNA1的作用机制与信号通路
2.1 突触信号传递机制
当运动神经元释放ACh时,两个ACh分子与CHRNA1结合,触发通道开放,产生终板电位并激活电压门控钠通道,引发肌肉收缩 [5]。其在突触后膜的聚集依赖agrin-LRP4-MuSK-rapsyn通路 [5]。在MuSK基因敲除小鼠中,CHRNA1水平下降80%,突触褶皱消失 [5]。
此外,PKA介导的磷酸化可增强受体敏感性,CaMKII则加速脱敏 [13]。在转录水平,neuregulin-1和MuSK信号促进CHRNA1表达及亚基转换。基因多态性(如rs16862847)与终板电位下降相关 [12]。在慢阻肺患者中,CHRNA1表达下调与气道收缩障碍相关 [11]。
2.2 与MuSK通路的关联
CHRNA1在agrin-MuSK通路中发挥核心作用。LRP4突变会削弱MuSK磷酸化及CHRNA1定位,导致先天性肌无力综合征(CMS)[3]。抗MuSK抗体同样可影响CHRNA1在突触膜的锚定 [1]。此外,CHRNA1表达变化还可能通过mTOR通路影响胸腺免疫耐受,提示其在神经-免疫交叉调控中的作用 [5]。
2.3 在mTOR通路中的调控
胸腺上皮细胞中,CHRNA1通过mTOR通路调控免疫耐受。其缺失会降低mTOR活性,导致胸腺发育障碍及自身免疫反应 [9][14]。CHRNA1与STAT3互作可激活Akt/mTOR通路,在niguding诱导的动脉粥样硬化模型中影响免疫功能 [15]。CHRNA1-mTOR轴过度激活可能与自身免疫性重症肌无力相关 [7]。
3. CHRNA1相关疾病
3.1 先天性肌无力综合征(CMS)
CHRNA1突变约占CMS的10%–15%,多为常染色体隐性遗传 [1]。错义和无义突变可降低受体对ACh的敏感性或表达水平,导致终板电位显著下降 [5]。患者常在婴儿期发病,表现为眼睑下垂、喂养困难、全身肌无力,且对吡啶斯的明反应有限 [16]。部分突变(如V285L)引起温度敏感性肌无力,机制可能与突变蛋白热不稳定性相关 [1]。
3.2 肺动脉高压(PAH)
CHRNA1可能通过调控线粒体电子传递链功能及ROS生成参与PAH。其在PAH患者肺组织中高表达,并与ETC复合物Ⅱ-Ⅲ活性降低相关 [10]。HIF-1α可上调CHRNA1转录 [11]。敲低CHRNA1可改善平滑肌细胞代谢异常与凋亡抵抗 [10]。动物模型显示,CHRNA1拮抗剂可降低肺动脉压力升高 [10]。
3.3 骨关节炎(OA)
OA患者滑膜中CHRNA1 mRNA显著上调,并与炎症因子IL-6、TNF-α正相关 [2]。多组学研究提示肠道菌群可通过“肠-肌肉-关节轴”调控CHRNA1表达,影响关节稳态 [17]。CHRNA1异常还与自噬障碍、线粒体损伤及软骨退变相关 [12][17]。其基因多态性与OA疼痛敏感性及病程进展有关 [8]。
4. CHRNA1靶点药物的最近研究进展
在疾病治疗方面,已有多种围绕CHRNA1的策略被提出。针对原发性局灶性多汗症,研究提示PAI1能够抑制CHRNA1表达,从而减少汗腺分泌;相反,其抑制剂PAI-039或PAI1基因敲除会增强与CHRNA1相关的多汗表型。而CHRNA1拮抗剂顺式阿曲库铵则可通过阻断离子通道缓解多汗症状。
对于CHRNA1基因突变导致的先天性肌无力综合征(CMS),靶向外显子P3A 5′剪接位点的反义寡核苷酸(AONs)被证明能够恢复正常剪接平衡,显示出潜在的治疗前景。此外,CHRNA1基因多态性还会影响患者对罗库溴铵等肌松药的敏感性及不良反应风险,为个体化用药提供了遗传学依据。
5. 华美生物CHRNA1研究相关产品
CHRNA1基因编码的乙酰胆碱受体α1亚基不仅是神经肌肉接头信号传递的核心分子,还通过与MuSK和mTOR通路的互作参与免疫调控和代谢调节。其突变或异常表达已被证实与CMS、PAH及OA等疾病密切相关。
华美生物提供多种CHRNA1相关蛋白和抗体产品,支持科研人员在分子机制研究和靶向治疗探索中的应用,助力推动CHRNA1在基础研究与临床转化中的进一步发展。
● CHRNA1重组蛋白
Recombinant Human Acetylcholine receptor subunit alpha (CHRNA1), partial; CSB-EP005386HU
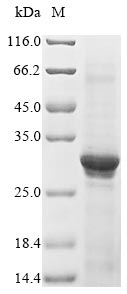
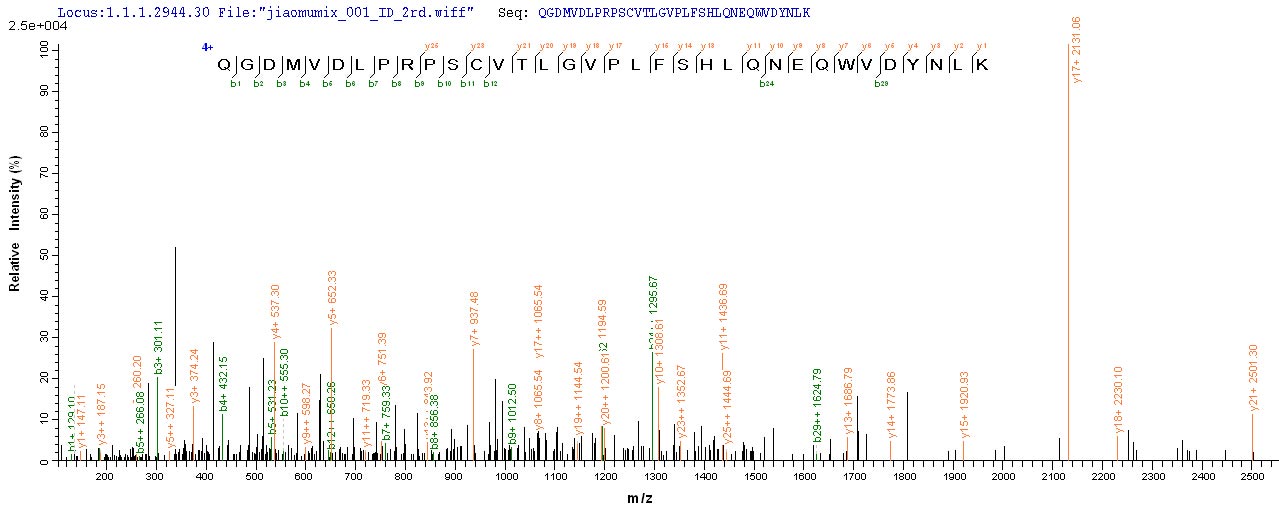
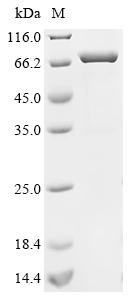
Recombinant Human Acetylcholine receptor subunit alpha (CHRNA1), partial, Biotinylated; CSB-EP005386HU-B
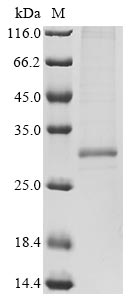
Recombinant Tetronarce californica Acetylcholine receptor subunit alpha (CHRNA1), partial; CSB-MP005386TOT
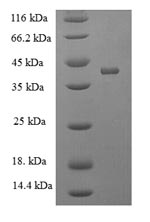
Recombinant Mouse Acetylcholine receptor subunit alpha (Chrna1), partial; CSB-EP005386MOa2
● CHRNA1抗体
CHRNA1 Antibody; CSB-PA11789A0Rb
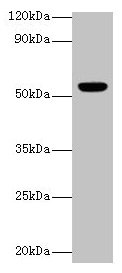
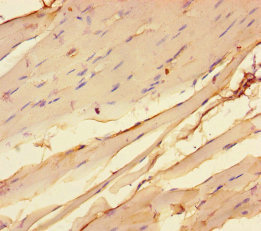
参考文献:
[1] Pedro M. Rodríguez Cruz, Jacqueline Palace, David Beeson. Congenital myasthenic syndromes and the neuromuscular junction[J]. Current Opinion in Neurology, 2014, 27(5): 566-575.
[2] Qin Chen, Shaosheng Bei, Zhiyun Zhang, Xiaofeng Wang, Yunying Zhu. Identification of diagnostic biomarks and immune cell infiltration in ulcerative colitis[J]. Scientific Reports, 2023, 13(1).
[3] Bisei Ohkawara, Macarena Cabrera‐Serrano, Tomohiko Nakata, Margherita Milone, Nobuyuki Asai, Kenyu Ito, Mikako Ito, Akio Masuda, Yasutomo Ito, Andrew G. Engel, Kinji Ohno. LRP4 third β-propeller domain mutations cause novel congenital myasthenia by compromising agrin-mediated MuSK signaling in a position-specific manner[J]. Human Molecular Genetics, 2013, 23(7): 1856-1868.
[4] Kai Qian, Jiaxin Xu, Yi Deng, Hao Peng, Jun Peng, Chun‐Mei Ou, Liu Zu, Lihong Jiang, Yonghang Tai. Signaling pathways of genetic variants and miRNAs in the pathogenesis of myasthenia gravis[J]. Gland Surgery, 2020, 9(6): 1933-1944.
[5] Bisei Ohkawara, Mikako Ito, Kinji Ohno. Secreted Signaling Molecules at the Neuromuscular Junction in Physiology and Pathology[J]. International Journal of Molecular Sciences, 2021, 22(5): 2455-2455.
[6] Matthieu Giraud, Richard Taubert, Claire Vandiedonck, Xiayi Ke, Matthieu Lévi‐Strauss, Franco Pagani, Francisco E. Baralle, B. Eymard, Christine Tranchant, Philippe Gajdos, Angela Vincent, Nick Willcox, David Beeson, Bruno Kyewski, Henri-Jean Garchon. An IRF8-binding promoter variant and AIRE control CHRNA1 promiscuous expression in thymus[J]. Nature, 2007, 448(7156): 934-937.
[7] Matthieu Giraud, Claire Vandiedonck, Henri‐Jean Garchon. Genetic Factors in Autoimmune Myasthenia Gravis[J]. Annals of the New York Academy of Sciences, 2008, 1132(1): 180-192.
[8] Peter Mu‐Hsin Chang, Yi‐Chen Yeh, Tzu-Chi Chen, Yu–Chung Wu, Pei‐Jung Lu, Hui-Chuan Cheng, Hsueh‐Ju Lu, Ming‐Huang Chen, Teh‐Ying Chou, Chi‐Ying F. Huang. High Expression of CHRNA1 is Associated with Reduced Survival in Early Stage Lung Adenocarcinoma after Complete Resection[J]. Annals of Surgical Oncology, 2013, 20(11): 3648-3654.
[9] Zhanfeng Liang, Lianjun Zhang, Huiting Su, Rong Luan, Ning Na, Lina Sun, Yang Zhao, Xiaodong Zhang, Qian Zhang, Juan Li, Lianfeng Zhang, Yong Zhao. MTOR signaling is essential for the development of thymic epithelial cells and the induction of central immune tolerance[J]. Autophagy, 2017, 14(3): 505-517.
[10] Xin Zhang, Jieling Li, Minyi Fu, Xijie Geng, Junjie Hu, Kejing Tang, Pan Chen, Jianyong Zou, Xiaoman Liu, Bo Zeng. Dysfunction in mitochondrial electron transport chain drives the pathogenesis of pulmonary arterial hypertension: insights from a multi-omics investigation[J]. Respiratory Research, 2025, 26(1).
[11] Lin Chen, Donglan Zhu, Jinfu Huang, Hui Zhang, Guang Zhou, Xiaoning Zhong. Identification of Hub Genes Associated with COPD Through Integrated Bioinformatics Analysis[J]. International Journal of COPD, 2022, Volume 17: 439-456.
[12] Patrick F. McArdle, Sue Rutherford, Braxton D. Mitchell, Coleen Damcott, Ying Wang, Ramachandran S. Vasan, Sandy Ott, Yen‐Pei C. Chang, Daniel Levy, Nanette Steinle. Nicotinic acetylcholine receptor subunit variants are associated with blood pressure
[13] Peter V. Lovell, David F. Clayton, Kirstin Replogle, Claudio V. Mello. Birdsong "Transcriptomics": Neurochemical Specializations of the Oscine Song System[J]. PLoS ONE, 2008, 3(10): e3440-e3440.
[14] Fatemeh Shirafkan, Luca Hensel, Kristin Rattay. Immune tolerance and the prevention of autoimmune diseases essentially depend on thymic tissue homeostasis[J]. Frontiers in Immunology, 2024, 15.
[15] Shuang Xu, Huaner Ni, Hangwei Chen, Qiuyan Dai. The interaction between STAT3 and nAChRα1 interferes with nicotine-induced atherosclerosis via Akt/mTOR signaling cascade[J]. Aging, 2019, 11(19): 8120-8138.
[16] Marco Calabrò, Laura Mandelli, Concetta Crisafulli, Antonella Sidoti, Tae‐Youn Jun, Soo-Jung Lee, Hans‐Jürgen Möller, Ashwin A. Patkar, Prakash S. Masand, Chi‐Un Pae, Alessandro Serretti. Genes Involved in Neurodevelopment, Neuroplasticity, and Bipolar Disorder: CACNA1C, CHRNA1, and MAPK1[J]. Neuropsychobiology, 2016, 74(3): 159-168.
[17] Tianyang Xu, Dong Kwon Yang, Kaiyuan Liu, Qiuming Gao, Zhongchen Liu, Guodong Li. Miya Improves Osteoarthritis Characteristics via the Gut-Muscle-Joint Axis According to Multi-Omics Analyses[J]. Frontiers in Pharmacology, 2022, 13.



