武汉华美生物工程有限公司CUSABIO®品牌商
15 年
手机商铺
- NaN
- 0
- 0
- 2
- 2
推荐产品
公司新闻/正文
巨噬细胞迁移抑制因子(MIF):连接炎症与肿瘤的多效性靶点
111 人阅读发布时间:2025-11-21 10:29
1. MIF的背景:结构、表达调控与基本生物学特性
巨噬细胞迁移抑制因子(MIF)最初因抑制巨噬细胞迁移而得名,现已被广泛认定为一种具有促炎和趋化特性的多效性细胞因子,可由多种免疫和非免疫细胞分泌,参与炎症、自身免疫和肿瘤等多种病理过程 [1-3]。
MIF独特之处在于其为糖皮质激素抗炎作用的内源性反调节因子 [3],在免疫平衡中居于核心地位。通过与CD74、CXCR2、CXCR4等受体结合,MIF形成复杂信号网络并介导多样生物学效应 [4-6]。
1.1 MIF的结构与催化活性
MIF独特的分子特征在于其具有内在的酮-烯醇互变异构酶活性,使其不同于多数经典细胞因子 [3]。MIF以三聚体形式存在,其活性位点结构赋予其催化潜能,能够参与多种炎症相关反应 [1]。其分子动力学特征与生物活性密切相关,对药物设计具有重要意义 [17]。
靶向MIF酶活性成为治疗研究重点。例如,KRP-6作为高效MIF酮酶抑制剂,可阻断M1巨噬细胞极化及氧化应激反应 [16]。而T-614(iguratimod)作为临床抗风湿药,通过变构方式抑制MIF三聚体,呈非竞争性动力学特征 [1,3]。此外,MIF-2也具有类似催化活性,其特异抑制剂4-CPPC可选择性阻断MIF-2与CD74结合,为家族功能研究提供工具 [10]。这些研究揭示MIF酶活与结构特征对其生物学作用至关重要。
1.2 表达调控与遗传变异
MIF的表达受多层调控,涉及转录、翻译及外界刺激反应。病原体相关分子模式(如LPS)可诱导MIF表达,表明其在先天免疫中作用显著 [18]。
转录因子ICBP90(UHRF1)通过结合MIF基因启动子区的−794 CATT重复序列调控其转录活性,该位点长度与MIF表达及糖皮质激素抵抗显著相关 [19]。此外,MIF启动子多态性(−173 G/C、−794 CATT)与阿尔茨海默病等疾病风险显著相关 [20]。这些变异可影响MIF的表达效率及个体对疾病的易感性 [21,22]。理解其遗传调控机制对于阐释MIF相关疾病的发病与药物反应具有重要价值。
1.3 基本生物学功能
MIF在细胞增殖、迁移及炎症反应中发挥核心作用。研究表明,重组MIF能在胃癌细胞中浓度依赖性促进细胞增殖并加速G1/S期转换 [23]。其机制涉及上调Cyclin D1、下调p27(Kip1),并激活PI3K/Akt信号通路。PI3K抑制剂LY294002可完全阻断这些效应 [23]。
这些发现表明,MIF通过调控PI3K/Akt-Cyclin D1轴促进细胞增殖,揭示其在肿瘤发展中的关键作用。
1.4 MIF家族成员MIF-2/DDT
MIF-2(DDT)是MIF家族的第二成员,与MIF-1共享酮-烯醇互变异构酶活性和受体结合特性 [10]。通过大规模化合物筛选发现4-CPPC可选择性抑制MIF-2酶活并阻断其与CD74结合,而不影响MIF-1-CD74信号 [10]。该研究揭示MIF-2在结构与功能上具有独特性,为开发高选择性靶向药物提供新方向。
2. MIF的受体与信号通路
2.1 MIF的受体及相互作用
2.1.1 CD74作为高亲和力受体
CD74原为MHC II伴侣分子,但同时也是MIF的高亲和力受体,可独立介导多种生物学功能 [24]。在活化的T细胞中,CD74显著上调并与CXCR4形成复合体参与MIF诱导的迁移 [4]。在多种肿瘤中,CD74-MIF信号通过ERK、AKT等通路促进细胞增殖、迁移及治疗抵抗 [12,29,30]。
在乳腺癌中,AEP-CD74轴激活ERK信号促进EMT,而抑制AEP和CD74可显著减少转移 [32]。在多发性骨髓瘤、胶质母细胞瘤等中,MIF/CD74信号上调与免疫逃逸及治疗耐受相关 [33-35]。此外,MIF/CD74轴在重症肌无力、子宫内膜异位症等疾病中同样具有调控作用 [36,37]。
2.1.2 CXCR2与CXCR4的趋化作用
MIF虽缺乏典型趋化因子结构,却能通过N-like环与伪ELR基序结合CXCR2及CXCR4 [38]。RLR序列在与CXCR4结合中起关键作用,其结合模式不同于CXCL12 [39]。MIF作为CXCR4的部分变构激动剂,能调节非经典G蛋白信号通路 [38,40]。MIF-CXCR4轴在动脉粥样硬化、神经母细胞瘤等疾病中促进细胞存活和迁移 [8],并与NFKB2通路共同参与急性髓系白血病耐药机制 [41]。此外,sCD74可通过抑制CXCR4-AKT轴诱导心肌细胞坏死性凋亡,在心衰中具有调节作用 [14]。
针对MIF-CXCR4的选择性抑制策略如肽类msR4M-L1,可特异阻断该通路而保留CXCL12-CXCR4保护作用,在动脉粥样硬化模型中表现出良好疗效 [42]。
2.2 MIF下游信号通路
MIF通过激活多条信号通路调控细胞增殖、炎症、代谢与免疫反应。主要通路包括ERK、JNK、NF-κB及PI3K/Akt等,其下游效应涵盖细胞存活、迁移、炎症因子释放及免疫极化等。
2.2.1 炎症与增殖信号
MIF结合CD74及共受体CD44后可激活ERK、JNK和NF-κB通路 [24,30]。在甲状腺癌中,抑制MIF/CD74内吞的4-IPP可激活JNK通路并诱导细胞凋亡,提示该轴在细胞存活与增殖调控中的重要性 [30]。
在乳腺癌转移模型中,AEP通过CD74激活ERK信号增强上皮-间质转化,而抑制AEP与CD74可显著抑制癌细胞迁移 [32]。在胶质母细胞瘤中,MIF/CD74信号抑制剂MN-166能降低ERK磷酸化并延长患者无进展生存期 [35]。此外,MIF-CD74轴的阻断可增强放疗诱导的M1极化反应,提高脑转移模型的放射敏感性 [29]。
κB通路亦为MIF炎症调控的核心。研究表明,FLT3突变型急性髓系白血病中,TKI治疗后MIF及CXCR2上调并激活非经典NFKB2通路。抑制NFKB2可显著下调MIF及相关炎症基因表达 [41]。
此外,小分子抑制剂CSB6B可通过促进MIF降解并抑制NF-κB活化,阻断破骨细胞分化,提示该通路在炎症性骨病中的作用 [43]。
在肺部组织中,WISP1可诱导MIF及其受体CD74、CD44的表达,通过Src激活EGFR并启动NF-κB、PI3K/Akt等多通路信号,促进炎症因子和重塑相关分子表达,揭示WISP1-MIF轴在气道炎症中的重要调控功能 [31]。
此外,MIF还参与类风湿关节炎的炎症放大与Th17细胞分化 [44],并介导慢性疼痛模型中的神经炎症反应 [45]。综上,MIF通过多层级信号网络调控细胞功能,形成炎症-增殖-迁移的病理环。
2.2.2 代谢调控与免疫分化
MIF不仅是炎症介质,也深度参与免疫代谢调控。在类风湿关节炎中,MIF促进Th17细胞分化,通过与ATF6直接结合增强ATF6通路活性,进而调控STAT3与RORC等基因表达,驱动Th17分化并加剧疾病进程 [44]。
在肿瘤微环境中,MIF通过影响巨噬细胞极化和能量代谢促进免疫逃逸。例如在骨肉瘤中,乳酸水平升高通过组蛋白H3K9乳酰化上调MIF表达,从而驱动巨噬细胞M2极化 [46]。MIF抑制剂4-IPP与PD-1抗体联合可显著抑制肿瘤生长,显示出免疫治疗协同潜力 [46]。
此外,MIF受雌激素-GPER通路调控,缺氧环境可诱导MIF与HIF-1α上调,而激活GPER能降低二者水平,提示MIF在内分泌应激适应中具关键作用 [47]。
2.3 与其他分子的互作
MIF可与其他趋化因子形成异源复合物以调节功能。例如,MIF与血小板来源的CXCL4L1形成高亲和力复合物,阻断MIF介导的T细胞趋化与血栓形成 [5]。这一复合物通过干扰MIF与CXCR4结合路径,起到内源性抑制作用 [5]。
此外,可溶性CD74(sCD74)可与MIF协同诱导心脏成纤维细胞坏死性凋亡。机制上,sCD74削弱MIF介导的AKT活化,促进p38通路激活并诱导RIP1/RIP3依赖性坏死。心力衰竭患者血清中sCD74/MIF比值显著降低,提示该轴具有潜在生物标志物意义 [14]。
这些研究表明,MIF通过与不同分子的互作精细调节信号强度与方向性,是其多效性的分子基础。
3. MIF在相关疾病中的作用
MIF广泛参与肿瘤、免疫、代谢及心血管疾病的病理过程,其促炎与促生存特性使其成为疾病进展的重要调控因子。
3.1 MIF与肿瘤发生发展
MIF在多种癌症中表现出促肿瘤活性。
在胃癌中,MIF促进细胞增殖与G1/S转化,通过上调Cyclin D1、下调p27并激活PI3K/Akt信号实现 [23]。在胰腺癌中,MIF抑制剂ISO-1可有效阻断肿瘤生长并抑制细胞迁移侵袭 [48]。在肝细胞癌中,MIF抑制与mTOR通路调节相关 [27]。
MIF在肿瘤侵袭与转移中亦发挥关键作用。神经母细胞瘤中,骨髓微环境诱导CXCR4上调,增强MIF信号及PI3K/AKT、ERK活性;抑制MIF可延缓肿瘤进展并提高化疗敏感性 [8]。乳腺癌中,AEP-CD74-ERK通路促进EMT,抑制该轴可显著抑制转移 [32]。
在骨肉瘤中,乳酸经组蛋白乳酰化增强MIF转录,驱动巨噬细胞M2极化;MIF抑制剂与PD-1抗体联合治疗可增强抗肿瘤免疫 [46]。GIST中MIF/CXCR4轴促进巨噬细胞极化并与复发风险相关 [7]。
MIF在非小细胞肺癌脑转移及头颈鳞癌中通过HIF与NF-κB/IL-6轴共同促进髓系细胞募集与血管生成 [6,29]。
此外,MIF/CD74信号在甲状腺癌、黑色素瘤及多发性骨髓瘤中上调,与细胞存活、治疗抵抗及免疫逃逸密切相关 [28,30,33,34]。
在临床应用方面,MIF血清水平在部分肿瘤中与预后相关。骨肉瘤患者治疗前MIF水平升高与较差疗效相关,治疗后下降提示预后改善 [51]。但在肺癌中,其单独作为生物标志物的效力有限 [25]。
这些研究表明,MIF通过调控细胞增殖、迁移及免疫微环境,系统性促进肿瘤发生发展,并可能成为多癌种通用治疗靶点。
3.2 炎症与自身免疫疾病
MIF是强效促炎因子,其在类风湿关节炎、哮喘及重症肌无力等疾病中均有上调 [1,15,37]。在RA中,MIF促进Th17分化并加剧炎症反应 [44];在过敏性哮喘模型中,MIF抑制剂SCD-19可有效缓解气道炎症与组织重塑 [15];在重症肌无力中,MIF-CD74信号增强B细胞存活并与疾病严重程度正相关 [37]。
MIF还参与急性肝损伤、慢性肾病及TAA诱导的肾毒性过程,表现为氧化应激和促纤维化反应增强 [1,2,11]。其抑制剂如iguratimod可显著提高肝损伤模型生存率并降低氧化应激 [3]。
在神经退行性疾病中,外周免疫细胞分泌的MIF可经CD74-CD44信号加重阿尔茨海默病病理 [13]。心血管系统中,sCD74/MIF比值下降与心力衰竭进展相关 [14]。这些结果表明,MIF在多系统炎症与免疫病理中发挥核心驱动作用。
4. MIF为药物靶点的研究进展
MIF(巨噬细胞移动抑制因子)作为重要的炎症与免疫调节靶点,相关药物研发呈现多样化趋势。目前已有小分子化药、抗体、PROTAC、基因治疗等多种类型药物在研,作用机制涵盖MIF抑制、CD74抑制、oxMIF靶向等。这些药物广泛探索于肿瘤、炎症性疾病、神经退行性疾病、纤维化等多个领域。全球多家机构参与研发,多数项目处于临床前阶段,仅异丁司特已获批上市,标志着该靶点药物研发正从基础研究向临床转化推进。
部分在研管线列举如下表:
| 药物 | 作用机制 | 药物类型 | 在研适应症 | 在研机构 | 最高研发阶段 |
|---|---|---|---|---|---|
| 异丁司特 | MIF抑制剂 | PDE10A抑制剂 | PDE11A抑制剂 | PDE3抑制剂 | PDE4抑制剂 | TLR4拮抗剂 | 小分子化药 | 哮喘 | 脑出血 | 脊髓型颈椎病 | 肌萎缩侧索硬化 | 新冠肺炎后遗症等 | KYORIN Pharmaceutical Co., Ltd. | The Ohio State University | MediciNova, Inc. | University of Pennsylvania | Portland VA Medical Center | 批准上市 |
| Imalumab | MIF抑制剂 | 免疫调节剂 | 单克隆抗体 | 腹水 | 结直肠癌 | Cytokine PharmaSciences, Inc. | 临床2期 |
| IPG-1094 | MIF抑制剂 | 小分子化药 | 狼疮性肾炎 | 局部晚期恶性实体瘤 | 黑色素瘤 | 肺癌脑转移 | 炎症性肠病 | 南京艾美斐生物医药科技股份有限公司 | 临床2期 |
| Fibrosis(Apaxen) | MIF抑制剂 | 化学药 | 纤维化 | Apaxen SA | 临床前 |
| 4-IPP | MIF抑制剂 | 小分子化药 | 急性髓性白血病 | Centre Hospitalier Universitaire Vaudois | 临床前 |
| MFC-1040 | MIF抑制剂 | NLRP3抑制剂 | 小分子化药 | 哮喘 | 特发性肺纤维化 | 肺动脉高压 | Sorbonne Paris Cité | Apaxen SA | Institut National de la Santé et de la Recherche Médicale | Université Paris-Saclay | Assistance Publique des Hôpitaux de Paris SA | Mifcare | 临床前 |
| INV-88 | MIF抑制剂 | 小分子化药 | 肿瘤 | 神经系统疾病 | 纤维化 | 血液肿瘤 | 类风湿关节炎 | 实体瘤 | Innovimmune Biotherapeutics, Inc. | 临床前 |
| RGB097 | MIF抑制剂 | 小分子化药 | 肿瘤 | University of Groningen | 临床前 |
| Zr89-ON102 | MIF抑制剂 | 诊断用放射药物 | 实体瘤 | OncoOne Research & Development GmbH | 临床前 |
| THOR-213 | CD74抑制剂 | MIF抑制剂 | ASO | 恶病质 | Thor Therapeutics Inc. | 临床前 |
| Hit-1(WuXi AppTec ) | MIF抑制剂 | 小分子化药 | 脓毒症 | 无锡药明康德新药开发股份有限公司 | 南京医科大学 | 临床前 |
| MD13 | MIF 降解剂 | 蛋白降解 | 蛋白水解靶向嵌合体(PROTAC) | 肺癌 | University of Groningen | 临床前 |
| Compound 37 (University of Pecs) | MIF抑制剂 | 小分子化药 | 脓毒性休克 | University of Pecs | 临床前 |
| ON05+Lu177-di-HSG | Histamine succinyl glycine抑制剂 | MFN2调节剂 | oxMIF抑制剂 | 双特异性抗体 | 治疗用放射药物 | 结直肠癌 | 头颈部肿瘤 | 胰腺癌 | 胃癌 | OncoOne Research & Development GmbH | 临床前 |
| ISO-1 | MIF抑制剂 | 小分子化药 | 前列腺炎 | 椎间盘退化 | 肌炎 | 罗斯河热 | 苏州大学 | University of Canberra | Griffith University | 安徽医科大学 | 江苏大学 | 临床前 |
| MIF-PROTAC(Princess Máxima Center) | MIF 降解剂 | 蛋白水解靶向嵌合体(PROTAC) | 神经母细胞瘤 | Prinses Máxima Centrum voor Kinderoncologie BV | 临床前 |
| PAANIB-1 | MIF抑制剂 | 化学药 | 帕金森病 | The Johns Hopkins University | 临床前 |
| ON-102 | oxMIF抑制剂 | 诊断用放射药物 | 炎症 | 实体瘤 | OncoOne Research & Development GmbH | 临床前 |
| P-EHC | MIF抑制剂 | 化学药 | 缺血 | 再灌注损伤 | 中国药科大学 | 临床前 |
| ON-203 | oxMIF抑制剂 | 单克隆抗体 | 结直肠癌 | 肺癌 | OncoOne Research & Development GmbH | 临床前 |
| Napa-001(NapaJen Pharma) | MIF抑制剂 | 寡核苷酸 | 溃疡性结肠炎 | 类风湿关节炎 | NapaJen Pharma, Inc. | 临床前 |
| ON-104 | oxMIF抑制剂 | 单克隆抗体 | 肾炎 | 哮喘 | 炎症性肠病 | 类风湿关节炎 | OncoOne Research & Development GmbH | 临床前 |
| DRalpha1-hMOG-35-55 (Virogenomics Biodevelopment) | CD74抑制剂 | HLA class II抗原调节剂 | MIF抑制剂 | 重组蛋白 | 多发性硬化症 | Virogenomics, Inc. | 临床前 |
| MFC-2040 | MIF抑制剂 | 小分子化药 | 肺动脉高压 | Mifcare | 临床前 |
| PAV-174 | MIF抑制剂 | 小分子化药 | 阿尔茨海默症 | Prosetta Biosciences, Inc. | 临床前 |
| AAV-PHP.eB-MIF-HA | MIF抑制剂 | 腺相关病毒基因治疗 | 肌萎缩侧索硬化 | - | 临床前 |
| M1 | MIF抑制剂 | 单克隆抗体 | 炎症 | Zavod Republike Slovenije ZA Transfuzijsko Medicino | 临床前 |
(数据截止到2025年11月8日,来源于synapse)
5. MIF研究工具
巨噬细胞迁移抑制因子(MIF)是一种具有多效性的细胞因子,在炎症、自身免疫性疾病、恶性肿瘤及多器官损伤中发挥关键作用。其独特的糖皮质激素反调节特性使其成为炎症及免疫调控的重要分子靶点。华美生物提供MIF重组蛋白、抗体及ELISA试剂盒产品,助力您进行相关机制研究及靶向药物开发。
● MIF重组蛋白
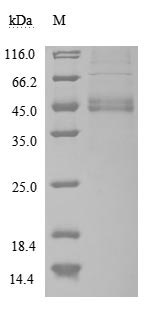
Recombinant Human Macrophage migration inhibitory factor (MIF) (Active); CSB-MP013826HU
● MIF抗体
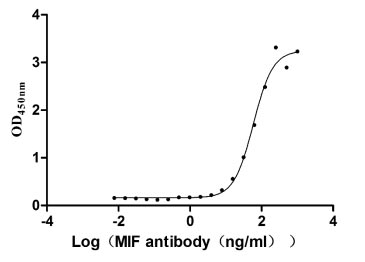
MIF Recombinant Monoclonal Antibody; CSB-RA146975A0HU
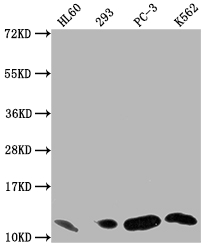
MIF Recombinant Monoclonal Antibody; CSB-RA013826MA1HU
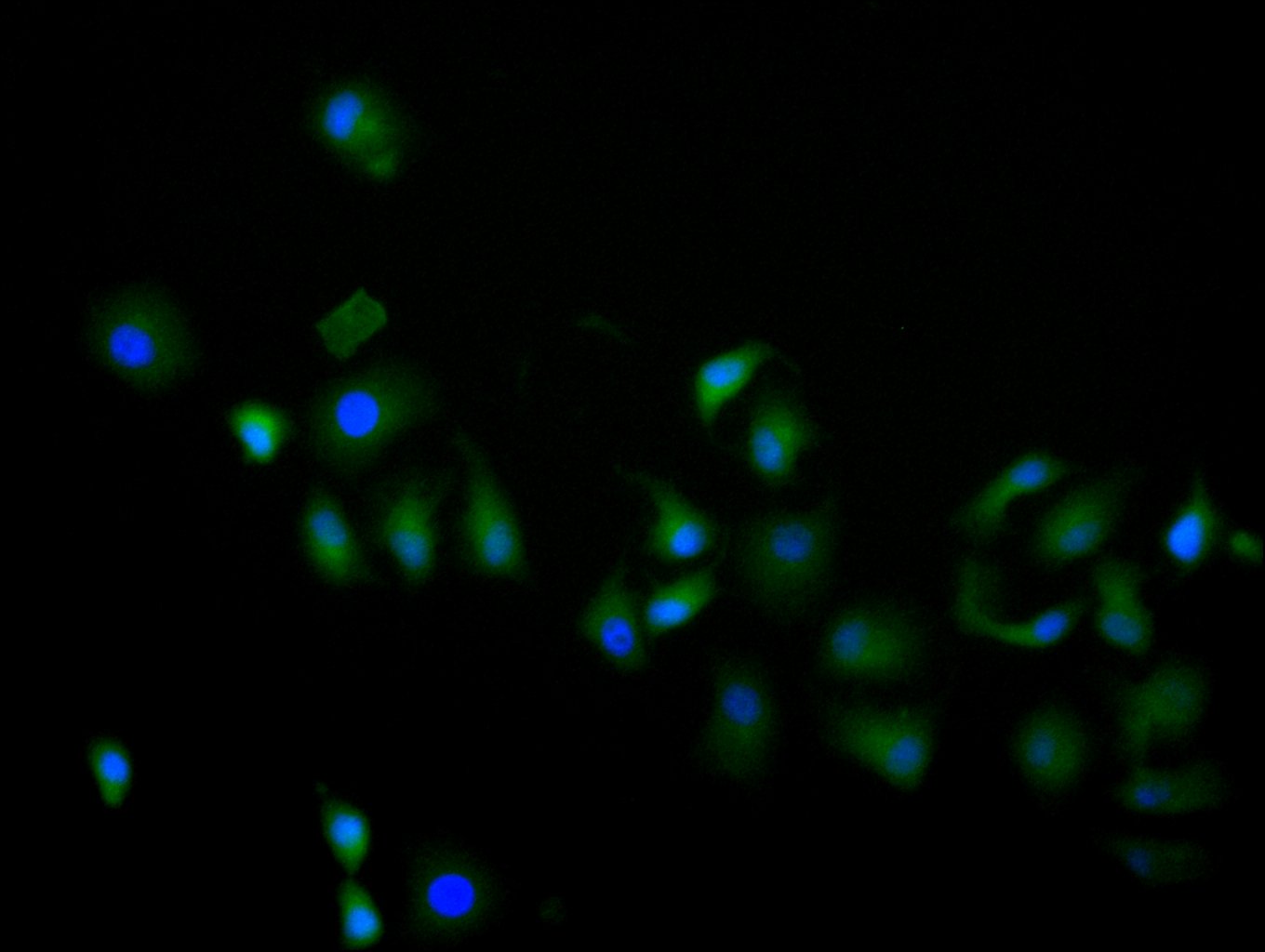
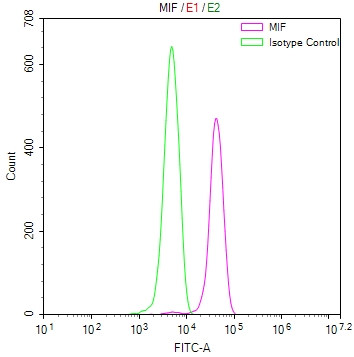
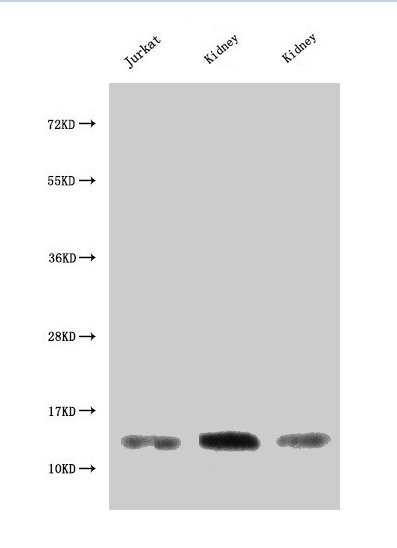
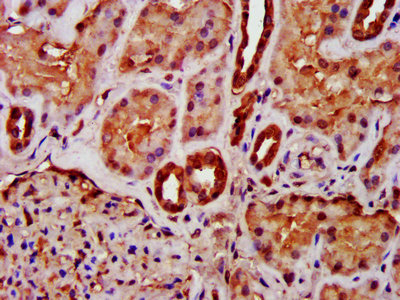
MIF Antibody; CSB-PA06867A0Rb
● MIF ELISA试剂盒

Human Macrophage Migration Inhibitory Factor,MIF ELISA Kit
CSB-E08330h
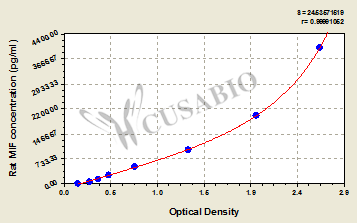
Rat Macrophage Migration Inhibitory Factor,MIF ELISA Kit
CSB-E07293r
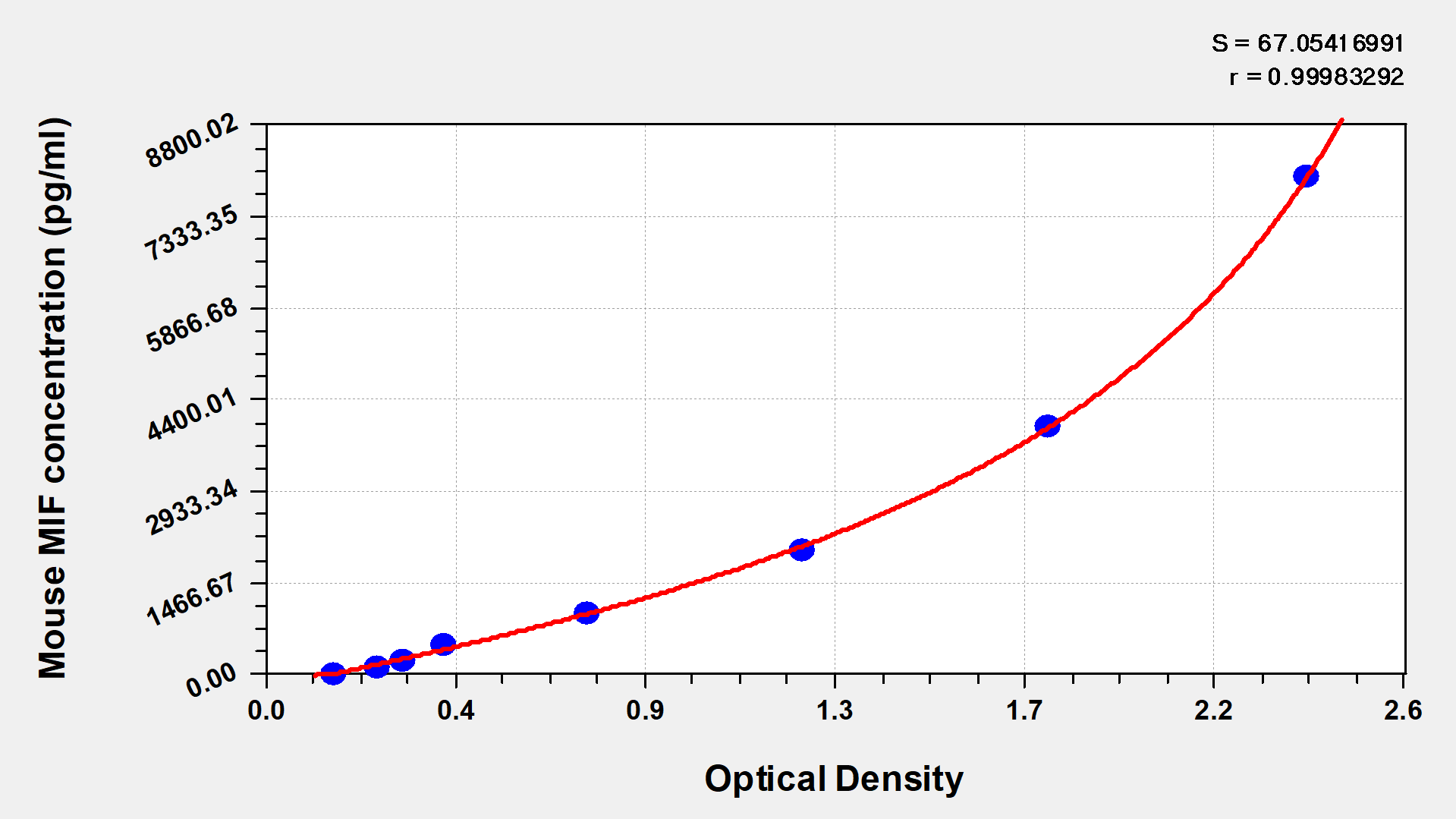
Mouse Macrophage Migration Inhibitory Factor,MIF ELISA Kit
CSB-E07292m
参考文献:
[1] Joshua Bloom, Georgios Pantouris, Mingzhu He, B. Aljabari, Lopa Mishra, Ramu Manjula, A. Parkins, E. Lolis, Yousef Al-Abed.(2024). Iguratimod, an allosteric inhibitor of macrophage migration inhibitory factor (MIF), prevents mortality and oxidative stress in a murine model of acetaminophen overdose.
[2] Bojan Jorgačević, S. Stanković, Jelena Filipović, J. Samardžić, D. Vučević, T. Radosavljević.(2022). Betaine modulates MIF-mediated oxidative stress, inflammation, and fibrogenesis in Thioacetamide-induced Nephrotoxicity.
[3] Joshua Bloom, C. Metz, Saisha A Nalawade, J. Casabar, K. Cheng, Mingzhu He, B. Sherry, T. Coleman, T. Forsthuber, Y. Al-Abed.(2016). Identification of Iguratimod as an Inhibitor of Macrophage Migration Inhibitory Factor (MIF) with Steroid-sparing Potential*.
[4] Lin Zhang, Iris Woltering, Mathias Holzner, Markus Brandhofer, Carl-Christian Schaefer, Genta Bushati, Simon Ebert, Bishan Yang, Maximilian Muenchhoff, J. Hellmuth, C. Scherer, Christian Wichmann, David Effinger, Max Hübner, O. El Bounkari, Patrick Scheiermann, J. Bernhagen, A. Hoffmann.(2024). CD74 is a functional MIF receptor on activated CD4+ T cells.
[5] Markus Brandhofer, A. Hoffmann, X. Blanchet, Elena Siminkovitch, Anne-Katrin Rohlfing, O. El Bounkari, Jeremy Nestele, Alexander Bild, Christos Kontos, Kathleen Hille, Vanessa Rohde, Adrian L. Fröhlich, Jona Golemi, O. Gokce, C. Krammer, P. Scheiermann, N. Tsilimparis, N. Sachs, W. Kempf, L. Maegdefessel, Michael K. Otabil, R. Megens, H. Ippel, R. Koenen, Junfu Luo, B. Engelmann, K. Mayo, M. Gawaz, A. Kapurniotu, C. Weber, P. von Hundelshausen, J. Bernhagen.(2021). Heterocomplexes between the atypical chemokine MIF and the CXC-motif chemokine CXCL4L1 regulate inflammation and thrombus formation.
[6] G. Zhu, Yaling Tang, Ning Geng, Min Zheng, Jian Jiang, Ling Li, Kaide Li, Zhengge Lei, Wei Chen, Yun-long Fan, Xiang-rui Ma, Longjiang Li, Xiaoyi Wang, Xin-hua Liang.(2014). HIF-α/MIF and NF-κB/IL-6 axes contribute to the recruitment of CD11b+Gr-1+ myeloid cells in hypoxic microenvironment of HNSCC.
[7] Shuo-meng Xiao, Rui Xu, Ying-xin Yang, Rui Zhao, Yuan Xie, Xu-dan Lei, Xiao-ting Wu.(2024). Gastrointestinal stromal tumors regulate macrophage M2 polarization through the MIF/CXCR4 axis to immune escape.
[8] Laura Garcia-Gerique, Marta García, Alícia Garrido-Garcia, Soledad Gómez-González, M. Torrebadell, E. Prada, Guillem Pascual-Pasto, Oscar Muñoz, S. Perez-Jaume, Isadora Lemos, Noelia Salvador, Mònica Vilà-Ubach, Ana Doncel-Requena, M. Suñol, A. Carcaboso, J. Mora, C. Lavarino.(2022). MIF/CXCR4 signaling axis contributes to survival, invasion, and drug resistance of metastatic neuroblastoma cells in the bone marrow microenvironment.
[9] D. Rodrigues, Elisa B. Prestes, Leandro de Souza Silva, A. Pinheiro, J. Ribeiro, A. Dicko, P. Duffy, M. Fried, I. Francischetti, E. Saraiva, Heitor A. Paula Neto, M. Bozza.(2019). CXCR4 and MIF are required for neutrophil extracellular trap release triggered by Plasmodium-infected erythrocytes.
[10] P. Tilstam, G. Pantouris, M. Corman, M. Andreoli, K. Mahboubi, G. Davis, Xin Du, L. Leng, E. Lolis, R. Bucala.(2019). A selective small-molecule inhibitor of macrophage migration inhibitory factor-2 (MIF-2), a MIF cytokine superfamily member, inhibits MIF-2 biological activity.
[11] A. Bruchfeld, J. Carrero, A. Qureshi, B. Lindholm, P. Bárány, O. Heimburger, M. Hu, Xinchun Lin, P. Stenvinkel, E. Miller.(2009). Elevated Serum Macrophage Migration Inhibitory Factor (MIF) Concentrations in Chronic Kidney Disease (CKD) Are Associated with Markers of Oxidative Stress and Endothelial Activation.
[12] Cintia D’Amato-Brito, Davide Cipriano, D. Colin, S. Germain, Y. Seimbille, J. Robert, F. Triponez, V. Serre-Beinier.(2016). Role of MIF/CD74 signaling pathway in the development of pleural mesothelioma.
[13] Bo Liu, Wei Luo, Ling Huang, Chun-ying Wei, Xiaorui Huang, Jun Liu, Ran Tao, Yingmin Mo, Xuebin Li.(2024). Migration Inhibition Factor Secreted by Peripheral Blood Memory B Cells Binding to CD74-CD44 Receptor Complex Drives Macrophage Behavior in Alzheimer’s Disease.
[14] Josefin Soppert, S. Kraemer, C. Beckers, Luisa Averdunk, J. Möllmann, B. Denecke, A. Goetzenich, G. Marx, J. Bernhagen, C. Stoppe.(2018). Soluble CD74 Reroutes MIF/CXCR4/AKT‐Mediated Survival of Cardiac Myofibroblasts to Necroptosis.
[15] H. Dunbar, I. Hawthorne, C. Tunstead, M. E. Armstrong, S. Donnelly, K. English.(2023). Blockade of MIF biological activity ameliorates house dust mite‐induced allergic airway inflammation in humanized MIF mice.
[16] Eszter Vámos, N. Kálmán, E. Sturm, B. Nayak, Julia Teppan, V. Vántus, D. Kovács, Lilla Makszin, Tamás Loránd, Ferenc Gallyas, Balázs Radnai.(2023). Highly Selective MIF Ketonase Inhibitor KRP-6 Diminishes M1 Macrophage Polarization and Metabolic Reprogramming.
[17] G. Pantouris, Junming Ho, Junming Ho, D. Shah, Syed, L. Leng, Vineet Bhandari, Vineet Bhandari, R. Bucala, Victor S. Batista, J. P. Loria, E. Lolis.(2018). Nanosecond Dynamics Regulate the MIF-Induced Activity of CD74.
[18] Laura La Paglia, M. Vazzana, M. Mauro, F. Dumas, A. Fiannaca, A. Urso, V. Arizza, A. Vizzini.(2023). Transcriptomic and Bioinformatic Analyses Identifying a Central Mif-Cop9-Nf-kB Signaling Network in Innate Immunity Response of Ciona robusta.
[19] Jie Yao, L. Leng, Weiling Fu, Jia Li, C. Bronner, R. Bucala.(2021). ICBP90 Regulates MIF Expression, Glucocorticoid Sensitivity, and Apoptosis at the MIF Immune Susceptibility Locus.
[20] Kübra Şahin, A. Rustemoglu.(2023). Investigation of MIF gene promoter variations and their haplotypes in the Alzheimer disease in Turkish population.
[21] C. J. Baños-Hernández, J. E. Navarro-Zarza, R. Bucala, J. Hernández-Bello, I. Parra-Rojas, M. G. Ramírez-Dueñas, S. García-Arellano, Luis Alexis Hernández-Palma, Andrea Carolina Machado-Sulbarán, J. Muñóz-Valle.(2019). Macrophage migration inhibitory factor polymorphisms are a potential susceptibility marker in systemic sclerosis from southern Mexican population: association with MIF mRNA expression and cytokine profile.
[22] N. Yazdani, M. Ashtiani, M. M. Zarandy, S. Mohammadi, H. Ghazavi, Elnaz Mahrampour, P. Amiri, M. Amoli.(2013). Association between MIF gene variation and Meniere’s disease.
[23] Guoqing Li, Juan Xie, Xiao-yong Lei, Li Zhang.(2009). Macrophage migration inhibitory factor regulates proliferation of gastric cancer cells via the PI3K/Akt pathway.
[24] R. Lindner.(2017). Invariant Chain Complexes and Clusters as Platforms for MIF Signaling.
[25] A. Rupp, Sophie Bahlmann, Nicolai Trimpop, J. von Pawel, S.Holdenrieder.(2023). Lack of clinical utility of serum macrophage migration inhibitory factor (MIF) for monitoring therapy response and estimating prognosis in advanced lung cancer.
[26] Nour K. Younis, Z. Solhjou, Hengcheng Zhang, Abdullah B. El Kurdi, Ahmad Halawi, R. Bucala, Dongliang Zhang, Jamil R. Azzi.(2023). MIF-CD74: A Novel Inflammatory Pathway that Suppresses Allograft-Infiltrating Tregs During Rejection.
[27] Shuai Lin, Qianwen Sheng, Xiao-bin Ma, Shanli Li, P. Xu, Cong Dai, Meng Wang, Huafeng Kang, Zhijun Dai.(2022). Marsdenia tenacissima Extract Induces Autophagy and Apoptosis of Hepatocellular Cells via MIF/mToR Signaling.
[28] K. Tanese, Y. Hashimoto, Z. Berková, Yuling Wang, F. Samaniego, Jeffrey E. Lee, S. Ekmekcioglu, E. Grimm.(2015). Cell Surface CD74-MIF Interactions Drive Melanoma Survival in Response to Interferon-γ.
[29] Lichao Liu, Jian Wang, Ying Wang, Lingjuan Chen, Ling Peng, Ya-wen Bin, Peng Ding, Ruiguang Zhang, Fan Tong, Xiaorong Dong.(2024). Blocking the MIF-CD74 axis augments radiotherapy efficacy for brain metastasis in NSCLC via synergistically promoting microglia M1 polarization.
[30] L. Varinelli, Dario Caccia, C. Volpi, C. Caccia, M. De Bortoli, E. Taverna, A. Gualeni, Valerio Leoni, A. Gloghini, G. Manenti, I. Bongarzone.(2015). 4-IPP, a selective MIF inhibitor, causes mitotic catastrophe in thyroid carcinomas.
[31] M. Christopoulou, S. Skandalis, Eleni Papakonstantinou, Daiana Stolz, A. Aletras.(2023). WISP1 induces the expression of macrophage migration inhibitory factor in human lung fibroblasts through Src kinases and EGFR-activated signaling pathways.
[32] Junsong Chen, Wenke Xu, Luyang Meng, Xin Zhang, Meng Lin, Sheng Zhang, Yi Liu, Fang Guo.(2025). The Combination of MIF Inhibitor and AEP Targeted Inhibitor to Reduce Lung Metastasis in Breast Cancer and Its Mechanism.
[33] Xinzhuo Chen, Renhua Huang, Huiping Wang, Hao Xiao, Qian Li, Zhimin Zhai, Zhitao Wang.(2024). Single Cell RNA-Seq Analysis Revealed MIF/CD74 Pathway Determinants of BCMA CART Resistance in Relapsed/Refractory Multiple Myeloma.
[34] Alessandro Canella, S. Rajendran, Matthew Nazzaro, Claire Schmitt, Daniel Kreatsoulas, Wesley Wang, P. Rajappa.(2024). IMMU-15. CD74/MIF SIGNALING AXIS DISRUPTION IN BONE MARROW-DERIVED MYELOID CELLS DELAYS MALIGNANT PROGRESSION IN A PRECLINICAL MODEL OF GLIOMA.
[35] Justin D. Lathia, Adam Lauko, Kazuko Matsuda, Malath Makhay, L. Nayak, U. Chukwueke, Eudocia Lee, D. Reardon, R. Beroukhim, Tracy T. Batchelor, E. Aquilanti, P. Wen.(2023). CTIM-36. IMMUNOHISTOCHEMISTRY EVALUATION ON PRE-TREATMENT TUMOR TISSUE PREDICTS TREATMENT RESPONSE TO MN-166 (IBUDILAST) AND TEMOZOLOMIDE COMBINATION THERAPY IN GLIOBLASTOMA PATIENTS.
[36] W. Nothnick, A. Graham.(2022). Dissecting the miR-451a-Mif Pathway in Endometriosis Pathophysiology Using a Syngeneic Mouse Model: Temporal Expression of Lesion Mif Receptors, Cd74 and Cxcr4.
[37] Paula Terroba-Navajas, I-Na Lu, Isaak Quast, M. Heming, C. Keller, L. Ostendorf, A. E. Hauser, R. Mothes, Helena Radbruch, Frauke Stascheit, Andreas Meisel, H. Wiendl, G. Meyer zu Hörste, Nick Willcox, Jan D. Lünemann.(2025). Single-Cell Transcriptomics Identifies a Prominent Role for the MIF-CD74 Axis in Myasthenia Gravis Thymus.
[38] D. Rajasekaran, Sabine Gröning, C. Schmitz, S. Zierow, Natalie Drucker, M. Bakou, Kristian Kohl, A. Mertens, H. Lue, C. Weber, Annie Xiao, G. Luker, A. Kapurniotu, E. Lolis, J. Bernhagen.(2016).
Macrophage Migration Inhibitory Factor-CXCR4 Receptor Interactions.
[39] M. Lacy, Christos Kontos, Markus Brandhofer, Kathleen Hille, Sabine Gröning, Dzmitry Sinitski, P. Bourilhon, E. Rosenberg, C. Krammer, T. Thavayogarajah, G. Pantouris, M. Bakou, C. Weber, E. Lolis, J. Bernhagen, A. Kapurniotu.(2018). Identification of an Arg-Leu-Arg tripeptide that contributes to the binding interface between the cytokine MIF and the chemokine receptor CXCR4.
[40] Cristina Perpiñá-Viciano, Ali Işbilir, Aurelien M. Zarca, B. Caspar, L. Kilpatrick, S. Hill, M. Smit, M. Lohse, C. Hoffmann.(2020). Kinetic Analysis of the Early Signaling Steps of the Human Chemokine Receptor CXCR4.
[41] H. Cao, Verena Tadros, Benjamin Hiramoto, Kevin Leeper, C. Hino, Jeffrey Xiao, Bryan Pham, Do Hyun Kim, M. Reeves, C. Chen, J. Zhong, Ke K. Zhang, Linglin Xie, Samiksha Wasnik, David J. Baylink, Yi Xu.(2022). Targeting TKI-Activated NFKB2-MIF/CXCLs-CXCR2 Signaling Pathways in FLT3 Mutated Acute Myeloid Leukemia Reduced Blast Viability.
[42] Christos Kontos, O. El Bounkari, C. Krammer, Dzmitry Sinitski, Kathleen Hille, C. Zan, Guangyao Yan, Sijia Wang, Ying Gao, Markus Brandhofer, R. Megens, A. Hoffmann, J. Pauli, Y. Asare, Simona Gerra, P.
Bourilhon, L. Leng, H. Eckstein, W. Kempf, J. Pelisek, O. Gokce, L. Maegdefessel, R. Bucala, M. Dichgans, C. Weber, A. Kapurniotu, J. Bernhagen.(2020). Designed CXCR4 mimic acts as a soluble chemokine receptor that blocks atherogenic inflammation by agonist-specific targeting.
[43] Kangtao Jin, Lin Zheng, Ziang Xie, L. Ye, Jiawei Gao, C. Lou, Wenzheng Pan, Bin Pan, Shijie Liu, Zhenzhong Chen, D. He.(2020). Chicago sky blue 6B (CSB6B), an allosteric inhibitor of macrophage migration inhibitory factor (MIF), suppresses osteoclastogenesis and promotes osteogenesis through the inhibition of the NF-κB signaling pathway.
[44] Guozhi Yan, Rongrong Song, Jieyu Zhang, Zhihao Li, Zhantao Lu, Zijian Liu, Xiaokang Zeng, Jie Yao.(2024). MIF promotes Th17 cell differentiation in rheumatoid arthritis through ATF6 signal pathway.
[45] Shaojing Ye, N. Agalave, Fei Ma, Dlovan F. D. Mahmood, Asma Al-Grety, P. E. Khoonsari, L. Leng, Camilla I. Svensson, R. Bucala, Kim Kultima, Pedro L. Vera.(2024). MIF-Modulated Spinal Proteins Associated with Persistent Bladder Pain: A Proteomics Study.
[46] Weidong Chen, Yan Liao, Hao Yao, Yutong Zou, Ji Fang, Jiongfeng Zhang, Zehao Guo, Jian Tu, Junkai Chen, Zijun Huo, Lili Wen, Xianbiao Xie.(2025). Multiomics integration analysis identifies tumor cell-derived MIF as a therapeutic target and potentiates anti-PD-1 therapy in osteosarcoma.
[47] Prosperl Ivette Wowui, Richard Mprah, Marie Louise Ndzie Noah, J. Adu-Amankwaah, Anastasia Wemaaatu Lamawura Kanoseh, Li Tao, Diana Chulu, Simon Kumah Yalley, Saffia Shaheen, Hong Sun.(2025). Estrogen via GPER downregulated HIF-1a and MIF expression, attenuated cardiac arrhythmias, and myocardial inflammation during hypobaric hypoxia.
[48] B. Cheng, Qiaofang Wang, Yaodong Song, Yanna Liu, Yanyan Liu, Shujun Yang, Dejian Li, Yan Zhang, Changju Zhu.(2020). MIF inhibitor, ISO-1, attenuates human pancreatic cancer cell proliferation, migration and invasion in vitro, and suppresses xenograft tumour growth in vivo.



