武汉华美生物工程有限公司CUSABIO®品牌商
15 年
手机商铺
- NaN
- 0
- 0
- 2
- 2
推荐产品
公司新闻/正文
CCL17:一种具有多生物学功能的趋化因子
279 人阅读发布时间:2025-06-13 09:55
1. CCL17概述
CCL17(也称为TARC)是一种属于CC亚家族的趋化因子,主要参与免疫细胞的迁移、激活和炎症反应的调节。它由树突状细胞、内皮细胞、角质形成细胞和成纤维细胞等产生,并在胸腺中高表达,对T细胞的发育、迁移和成熟T细胞的激活起重要作用。CCL22和CCL17同属于CC型趋化因子家族,二者具有高度同源性,具有共同的受体分子CCR4,都可以有效趋化CCR4表达阳性的细胞。
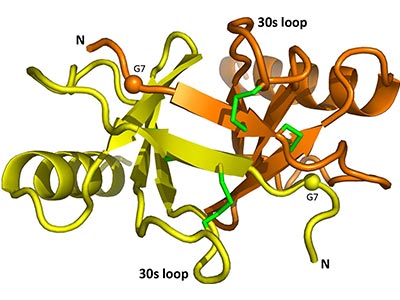
Figure 1. CCL17二聚体结构,来源于PDB:1NR4。球体部分为G7T突变位置,绿色部分为二硫键位置。
来源:DOI: 10.1016/j.bbrep.2017.11.005
2. CCL17与疾病的关联
2.1 心血管疾病
CCL17在心血管疾病中起着关键作用,尤其是在年龄相关和血管紧张素II (Ang II)诱导的病理性心脏重塑和心力衰竭中。研究发现,随着年龄的增长,血清中CCL17水平显著增加,并与心脏功能障碍相关。在动物实验中,CCL17基因敲除小鼠显示出对年龄和Ang II诱导的心脏肥大和纤维化的抑制作用,并伴随着T细胞亚群的可塑性和分化 [1]。此外,使用抗CCL17中和抗体的治疗也显著抑制了Ang II诱导的病理性心脏重塑 [2]。这些发现揭示了CCL17作为一种新的治疗靶点的潜力,可用于年龄相关和Ang II诱导的病理性心脏肥大和心力衰竭。
2.2 炎症与自身免疫性疾病
趋化因子受体CCR4是CCL17的高亲和力受体,CCL17在炎症中的作用主要体现在其通过与CCR4结合,参与调控炎症反应和炎症性疼痛。在炎症模型中,CCL17能够促进炎症性T细胞的趋化和激活,尤其是在Th2型免疫反应中 [3]。在骨关节炎模型中,CCL17基因敲除小鼠显示出对疼痛和疾病发展的抵抗性,表明CCL17在炎症性疼痛的发病机制中起着关键作用 [4]。此外,在炎症性关节炎和肠道炎症模型中,CCL17的缺乏也能够减轻疾病症状,进一步支持了CCL17在炎症中的重要作用 [5][6]。
在自身免疫疾病中,CCL17同样扮演着重要角色 [3]。在系统性红斑狼疮(SLE)中,CCL17的表达也有所增加,提示其可能参与SLE的免疫病理过程 [7][8]。此外,在多发性硬化症(MS)的动物模型实验性自身免疫性脑脊髓炎(EAE)中,CCL17基因敲除小鼠显示出减轻的疾病症状 [9]。这些研究结果表明,CCL17在自身免疫疾病的发病机制中具有重要作用,并可能成为治疗这些疾病的新靶点。
CCL17在过敏性哮喘中发挥着关键作用,它由树突状细胞(DCs)产生,能吸引Th2细胞到气道,引发过敏性哮喘的炎症反应。NOD1刺激的树突状细胞在体内能加重Th2肺部反应,且这种作用以CCL17依赖性方式发生 [10]。通过人源化SCID小鼠模型的研究发现,阻断CCR4(CCL17的受体)能显著减少气道炎症和支气管高反应性,表明CCR4-CCL17轴在Th2细胞招募到气道中起到关键作用 [11]。这些研究结果都暗示了CCL17可能是一个潜在的治疗过敏性哮喘的靶点。
2.3 肿瘤及肿瘤微环境
CCL17在多种肿瘤细胞中高表达,能促进肿瘤细胞增殖、迁移和侵袭。在肝细胞癌中,与M2型巨噬细胞共培养或用CCL17处理,可增强肿瘤细胞的这些恶性生物学行为,还能提升细胞干性,促进肿瘤球形成,并上调肿瘤干细胞相关转录因子表达,助力肿瘤复发和转移 [12]。此外,CCL17通过激活TGF-β1/Smad和Wnt/β-catenin信号通路,影响上皮-间质转化过程,进一步加强肿瘤细胞的侵袭和转移能力 [13]。
CCL17与多种免疫细胞浸润相关。在胃癌和甲状腺癌中,其高表达与Foxp3+调节性T细胞聚集有关,可能抑制抗肿瘤免疫 [13][14]。同时,CCR4-CCL17信号轴选择性招募Th2细胞、Tregs、记忆T细胞至炎症或肿瘤微环境,通过JAK/STAT6、PI3K/AKT通路激活免疫抑制程序。
3. 相关信号通路研究
| 信号通路/分子 | 作用描述 | 相关疾病/细胞类型 | 参考文献 |
|---|---|---|---|
| CCL17/CCR4 | 促进肿瘤细胞迁移、侵袭、干性维持,调控免疫细胞迁移 | 多种肿瘤、干细胞 | 12, 15, 16, 17, 18 |
| ERK/PD-L1 | CCL17通过CCR4激活ERK/PD-L1信号,增强肿瘤细胞恶性行为 | 食管鳞癌 | 15 |
| mTORC1/mTORC2 | 乳酸诱导M2极化,M2分泌CCL17,CCL17/CCR4激活mTORC1促进肿瘤侵袭 | 垂体腺瘤 | 16, 19 |
| Wnt/β-catenin、TGF-β1 | CCL17促进肿瘤细胞EMT及干性,激活Wnt/β-catenin和TGF-β1信号 | 肝细胞癌 | 12 |
| STAT6/IRF4/JMJD3 | IL-4诱导CCL17表达依赖STAT6-IRF4-JMJD3通路,涉及表观遗传调控 | 单核细胞/巨噬细胞 | 7 |
| β-arrestin | CCL17激活CCR4主要招募β-arrestin而非G蛋白,提示其可能作为清道夫受体 | T细胞 | 20 |
4. 临床意义与应用前景
4.1 生物标志物与预后预测
CCL17/CCL22高表达可预测头颈鳞癌患者的免疫检查点抑制剂疗效及生存率 [19],在鼻型NK/T细胞淋巴瘤、肝癌等疾病中也有潜在的诊断和预后价值 [12][18]。
4.2 靶向治疗潜力
抗CCR4单抗可介导NK细胞杀伤肿瘤细胞,CCL17/CCR4轴成为多种肿瘤的新型靶向治疗候选 [18][21]。
5. 相关产品推荐
华美生物提供CCL17相关高质量重组蛋白和抗体,旨在帮助科研工作者进行CCL17作用机制与临床转化方向的研究:
● CCL17重组蛋白
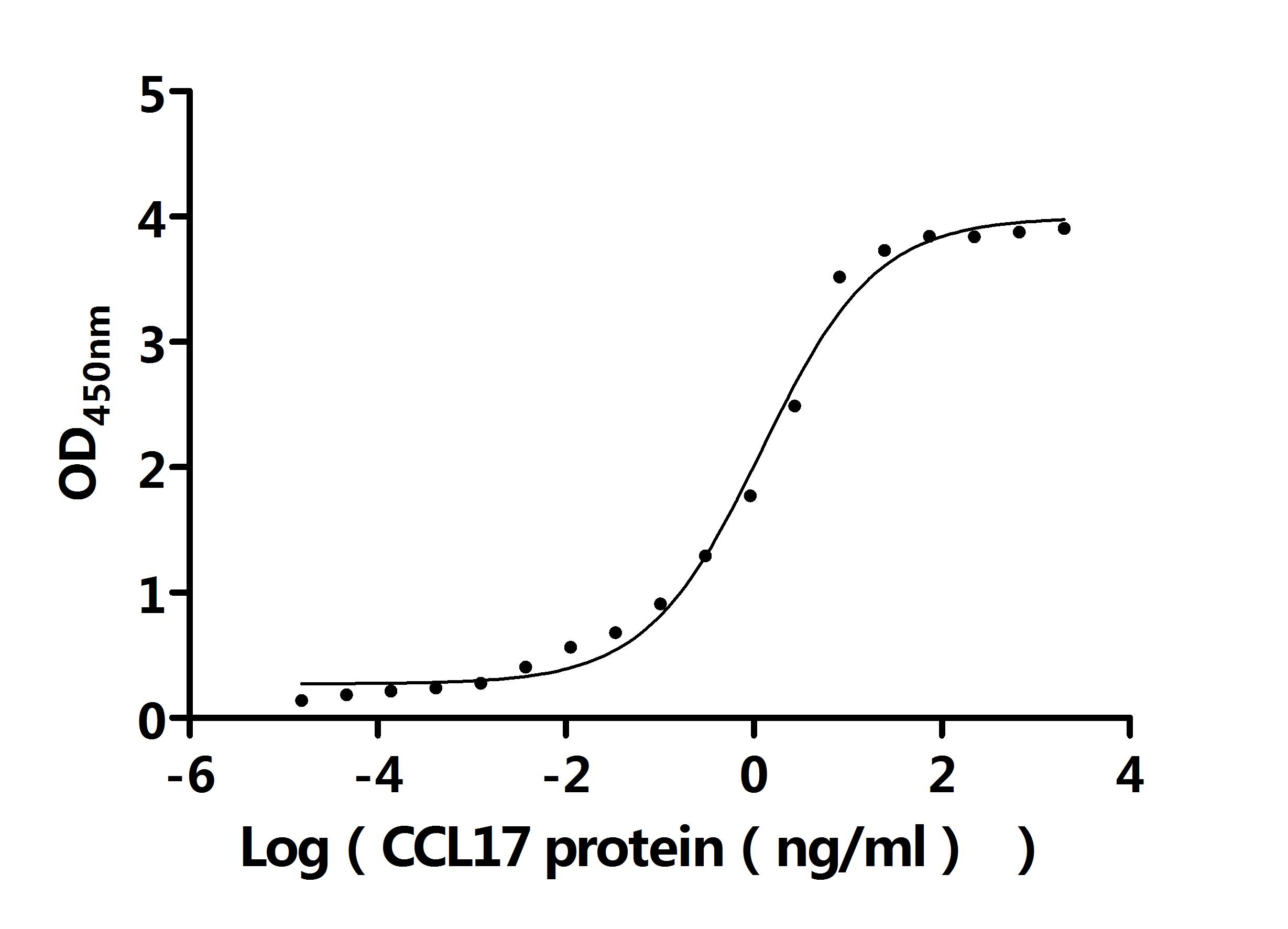
Recombinant Human C-C motif chemokine 17 (CCL17) (Active); CSB-MP856406HU
Recombinant Macaca mulatta C-C motif chemokine 17 (CCL17) (Active); CSB-MP811562MOW
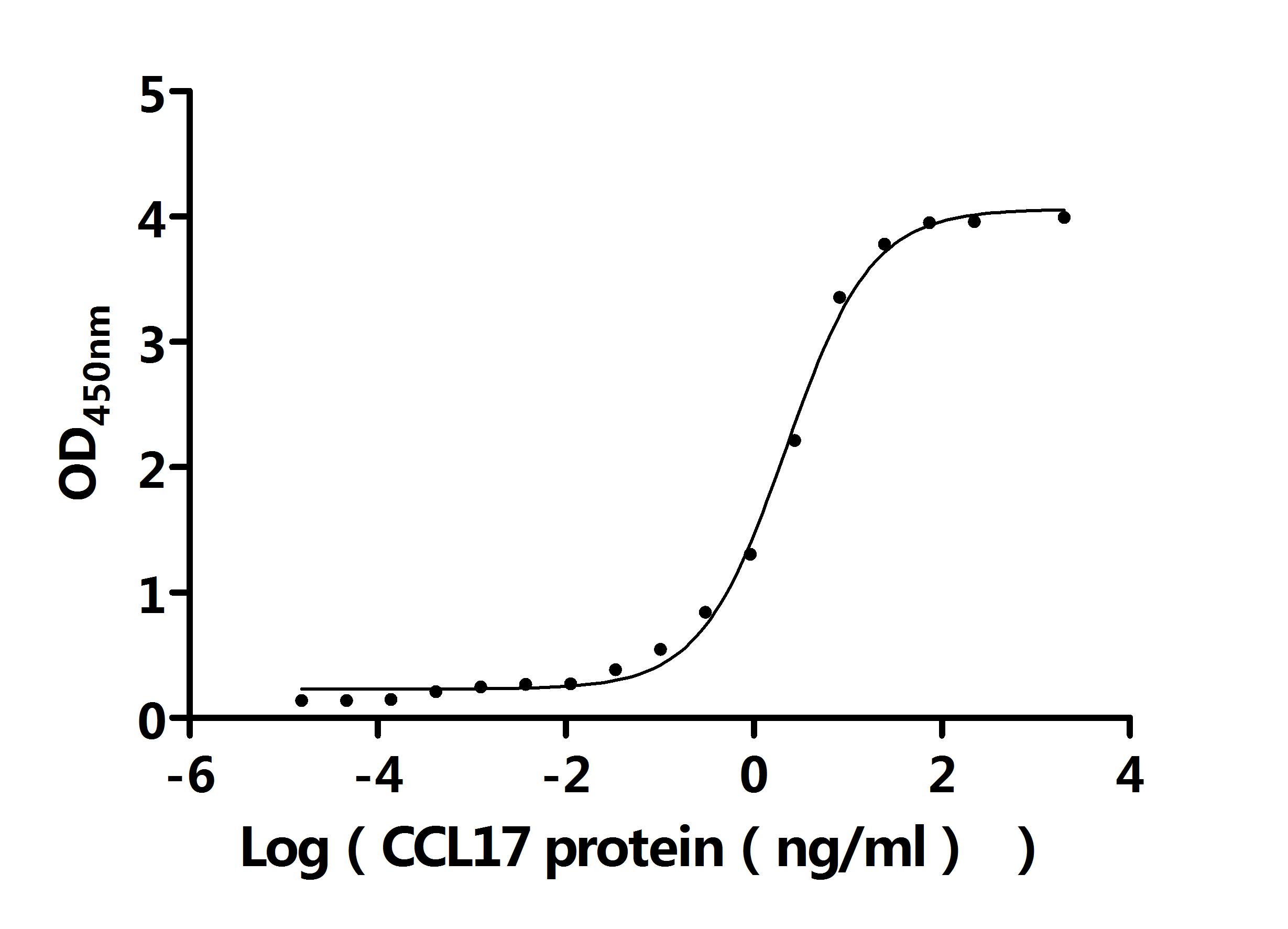
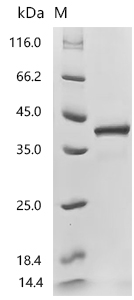
Recombinant Mouse C-C motif chemokine 17 (Ccl17) (Active); CSB-MP6512MO
● CCL17重组抗体
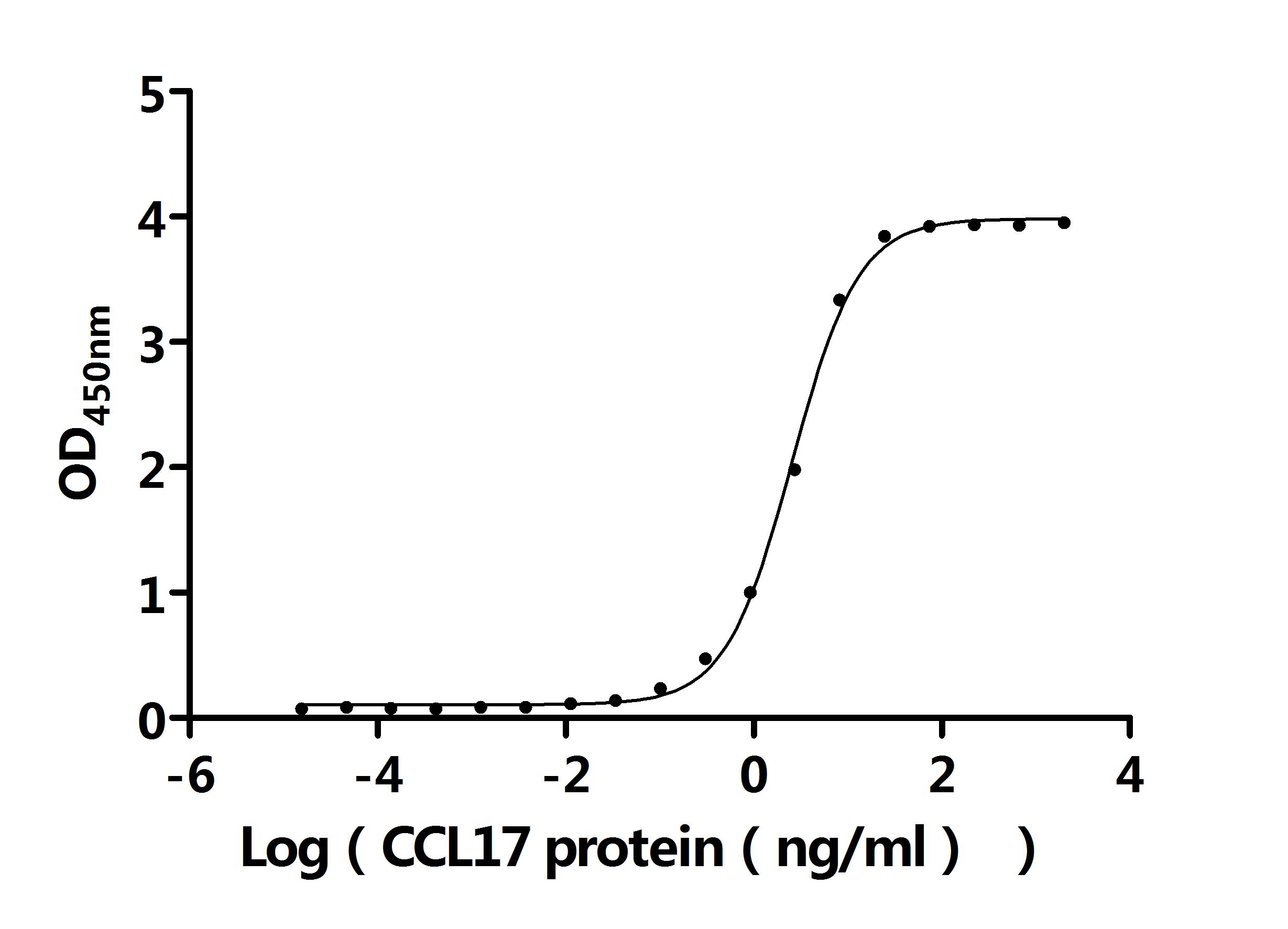
CCL17 Recombinant Monoclonal Antibody; CSB-RA856406MA1HU
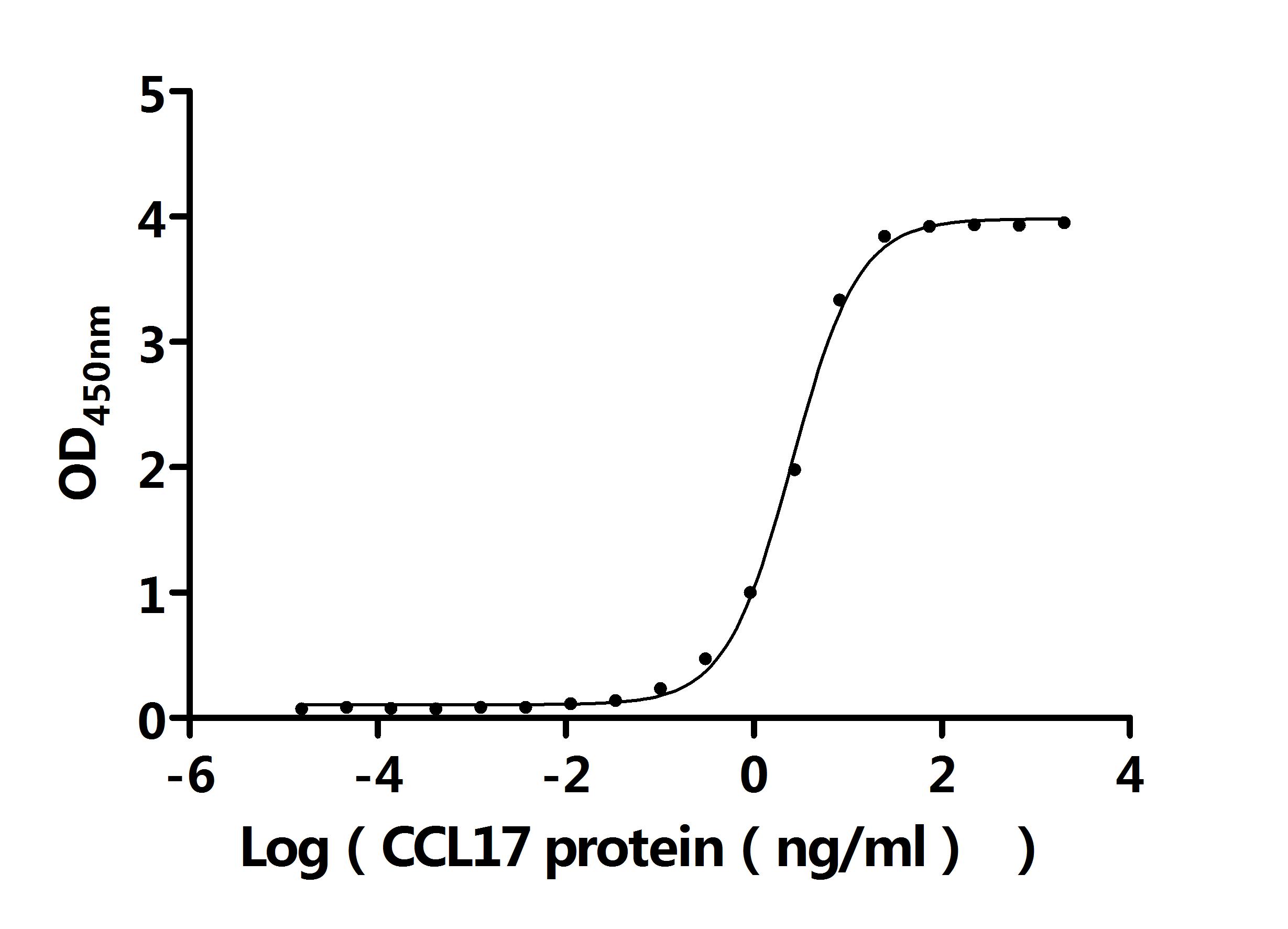
Activity: Measured by its binding ability in a functional ELISA. Immobilized Anti-CCL17 recombinant antibody at 2 μg/mL can bind Human CCL17 protein (CSB-MP856406HU). The EC50 is 2.403-2.741 ng/mL.
● CCL17(TRAC) ELISA试剂盒
Human thymus activation regulated chemokine,TARC/CCL17 ELISA Kit; CSB-E09257h
Monkey C-C motif chemokine 17(CCL17) ELISA kit; CSB-EL004780RH
Canine thymus activation regulated chemokine(TARC/CCL17)ELISA Kit; CSB-E17792c
参考文献:
[1] Zhang, Y., Ye, Y., Tang, X., Wang, H., Tanaka, T., Tian, R., Yang, X., Wang, L., Xiao, Y., Hu, X., Jin, Y., Pang, H., Du, T., Liu, H., Sun, L., Xiao, S., Dong, R., Ferrucci, L., Tian, Z., & Zhang, S. (2022). CCL17 acts as a novel therapeutic target in pathological cardiac hypertrophy and heart failure. The Journal of Experimental Medicine, 219(8).
[2] Zhang, Y., Tang, X., Wang, Z., Wang, L., Chen, Z., Qian, J. Y., Tian, Z., & Zhang, S. Y. (2023). The chemokine CCL17 is a novel therapeutic target for cardiovascular aging. Signal transduction and targeted therapy, 8(1), 157.
[3] Lupancu, T.J., Eivazitork, M., Hamilton, J.A., Achuthan, A.A. and Lee, K.M.-C. (2023), CCL17/TARC in autoimmunity and inflammation—not just a T-cell chemokine. Immunol Cell Biol, 101: 600-609.
[4] Lee, M.-C., Saleh, R., Achuthan, A., Fleetwood, A. J., Förster, I., Hamilton, J. A., & Cook, A. D. (2018). CCL17 blockade as a therapy for osteoarthritis pain and disease. Arthritis Research & Therapy, 20(1), 62.
[5] Heiseke, A. F., Faul, A. C., Lehr, H., Förster, I., Schmid, R. M., Krug, A. B., & Reindl, W. (2011). CCL17 Promotes Intestinal Inflammation in Mice and Counteracts Regulatory T Cell–Mediated Protection From Colitis. Gastroenterology, 142(2), 335–345.
[6] Achuthan, A., Cook, A. D., Kevin M.-C. Lee, Saleh, R., Hsu Wei Khiew, Melody Wei-Ning Chang, Louis, C., Fleetwood, A. J., Lacey, D., Anne Deen Christensen, Frye, A. T., Pui Yeng Lam, Kusano, H., Nomura, K., Steiner, N., Förster, I., Nutt, S. L., Olshansky, M., Turner, S. J., & Hamilton, J. A. (2016). Granulocyte macrophage colony-stimulating factor induces CCL17 production via IRF4 to mediate inflammation. Journal of Clinical Investigation, 126(9), 3453–3466.
[7] Hsu, A. T., Lupancu, T. J., Lee, M.-C., Fleetwood, A. J., Cook, A. D., Hamilton, J. A., & Achuthan, A. (2018). Epigenetic and transcriptional regulation of IL4-induced CCL17 production in human monocytes and murine macrophages. Journal of Biological Chemistry, 293(29), 11415–11423.
[8] T R D J Radstake. (2004). Increased expression of CCL18, CCL19, and CCL17 by dendritic cells from patients with rheumatoid arthritis, and regulation by Fc gamma receptors. Annals of the Rheumatic Diseases, 64(3), 359–367.
[9] Dhaiban, S., Al-Ani, M., Elemam, N. M., & Maghazachi, A. A. (2020). Targeting Chemokines and Chemokine Receptors in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Journal of Inflammation Research, Volume 13, 619–633.
[10] Yahia, S. A., Imane Azzaoui, Laetitia Everaere, Vorng, H., Cécile Chenivesse, Philippe Marquillies, Duez, C., Delacre, M., Grandjean, T., Balsamelli, J., d’Andon, M. F., Fan, Y., Coline Ple, Werts, C., Ivo Gomperts Boneca, Wallaert, B., Mathias Chamaillard, & Tsicopoulos, A. (2014). CCL17 Production by Dendritic Cells Is Required for NOD1-mediated Exacerbation of Allergic Asthma. American Journal of Respiratory and Critical Care Medicine, 189(8), 899–908.
[11] Perros, F., Hoogsteden, H. C., Coyle, A. J., Lambrecht, B. N., & Hammad, H. (2009). Blockade of CCR4 in a humanized model of asthma reveals a critical role for DC-derived CCL17 and CCL22 in attracting Th2 cells and inducing airway inflammation. Allergy, 64(7), 995–1002.
[12] Zhu, F., Li, X., Chen, S., Zeng, Q., Zhao, Y., & Luo, F. (2016). Tumor-associated macrophage or chemokine ligand CCL17 positively regulates the tumorigenesis of hepatocellular carcinoma. Medical Oncology, 33(2).
[13] Mizukami, Y., Kono, K., Kawaguchi, Y., Akaike, H., Kamimura, K., Sugai, H., & Fujii, H. (2008). CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. International Journal of Cancer, 122(10), 2286–2293.
[14] Gu, X., Chen, B., Zhang, S., Zhai, X., Hu, Y., & Ye, H. (2024). The expression of CCL17 and potential prognostic value on tumor immunity in thyroid carcinoma based on bioinformatics analysis. Scientific Reports, 14(1).
[15] Jin, C., Lu, L., Gao, J., & Chen, L. (2024). M2-like Macrophages-derived CCL17 Promotes Esophageal Squamous Cell Carcinoma Metastasis and Stemness via Activating CCR4-mediated ERK/PD-L1 Pathway.. Current molecular medicine.
[16] Zhang, A., Xu, Y., Xu, H., Ren, J., Meng, T., Ni, Y., Zhu, Q., Zhang, W., Pan, Y., Jin, J., Bi, Y., Wu, Z., Lin, S., & Lou, M. (2021). Lactate-induced M2 polarization of tumor-associated macrophages promotes the invasion of pituitary adenoma by secreting CCL17. Theranostics, 11, 3839 - 3852.
[17] Konno, K., Sasaki, T., Kulkeaw, K., & Sugiyama, D. (2020). Paracrine CCL17 and CCL22 signaling regulates hematopoietic stem/progenitor cell migration and retention in mouse fetal liver.. Biochemical and biophysical research communications.
[18] Kumai, T., Nagato, T., Kobayashi, H., Komabayashi, Y., Ueda, S., Kishibe, K., Ohkuri, T., Takahara, M., Celis, E., & Harabuchi, Y. (2015). CCL17 and CCL22/CCR4 signaling is a strong candidate for novel targeted therapy against nasal natural killer/T-cell lymphoma. Cancer Immunology, Immunotherapy, 64, 697-705.
[19] Zhou, W., Zhang, X., Feng, Y., Zhang, Y., & Liu, Z. (2022). The CC ligand chemokine family members CCL17/CCL22 predict the survival and response to immune checkpoint blockade therapy of patients with head and neck squamous cell carcinoma.. Current problems in cancer, 46 6, 100896 .
[20] Lim, H., Lane, J., Canals, M., & Stone, M. (2021). Systematic Assessment of Chemokine Signaling at Chemokine Receptors CCR4, CCR7 and CCR10. International Journal of Molecular Sciences, 22.
[21] Goenka, A., Khan, F., Verma, B., Sinha, P., Dmello, C., Jogalekar, M., Gangadaran, P., & Ahn, B. (2023). Tumor microenvironment signaling and therapeutics in cancer progression. Cancer Communications, 43, 525 - 561.



