武汉华美生物工程有限公司CUSABIO®品牌商
15 年
手机商铺
- NaN
- 0
- 0
- 2
- 2
推荐产品
公司新闻/正文
SLC7A11:SLC家族氨基酸转运蛋白,抗铁死亡!肿瘤耐药新方向!
2135 人阅读发布时间:2022-10-19 16:53
近期,Cell 杂志推出重磅“铁死亡”综述,纪念铁死亡十周年 [1]!该文章主要介绍了铁死亡的关键调控因子,以及未来利用铁死亡来促进治疗方面的前景。SLC7A11作为铁死亡的最关键的上游调节因子之一,近年来,大量文献早已揭示SLC7A11驱动铁死亡抗性,在肿瘤等疾病中发挥调控作用。SLC7A11不仅是铁死亡的有效靶标,在多种肿瘤耐药的治疗中,SLC7A11也扮演重要的角色,包括肺腺癌、胃癌、结直肠癌、胶质瘤等等。到目前为止,研究学者已经探索多种抗癌方法,然而,有效治疗的一个主要障碍是对抗癌治疗的适应性耐药性。SLC7A11对于这一障碍所带来的影响,有望提供走出困境的替代方案。那么,SLC7A11是什么家族蛋白?SLC7A11在铁死亡及肿瘤耐药中的机制又如何?今天我们一起来了解一下。
1. 什么是溶质转运蛋白SLC?
2. 什么是SLC7A11?
3. SLC7A11相关的调节机制
- 3.1 SLC7A11和Xc-系统
- 3.2 SLC7A11与铁死亡
- 3.3 SLC7A11与肿瘤耐药
4. SLC7A11在肿瘤靶向治疗中的作用
- 4.1 SLC7A11与神经系统肿瘤
- 4.2 SLC7A11与乳腺癌及生殖系统肿瘤
- 4.3 SLC7A11与呼吸系统肿瘤
- 4.4 SLC7A11与消化系统肿瘤
5. SLC7A11的临床应用前景
1. 什么是溶质转运蛋白SLC?
溶质载体(Solute Carriers, SLC),即溶质转运蛋白超家族是人类细胞膜(含胞内膜)上最重要的膜转运蛋白家族之一 [2]。目前已鉴定的人源SLC转运蛋白超家族系列包含52个亚家族,共400多个成员 [2, 3]。SLC转运蛋白超家族是仅次于G蛋白偶联受体(GPCR)后的第二大膜蛋白家族 [4]。SLCs亚细胞分布广泛,除可分布于细胞膜外,还可分布在细胞核膜、内质网、线粒体、溶酶体、高尔基体和过氧化物酶体等细胞器 [4, 5]。SLC作为典型的跨膜蛋白,介导各种营养物质和代谢产物的跨膜生物膜转运,包括金属离子、无机离子、有机离子、氨基酸、脂质、糖类、神经递质、核酸和药物等 [6-8] (图1)。
研究表明,SLCs蛋白表达异常或功能缺陷与多种疾病的发生发展密切相关,包括肿瘤、代谢性疾病、心血管疾病、免疫系统和神经功能障碍等等,使得该家族蛋白的功能研究近年来备受关注 [7]。尤其在肿瘤中,SLC的成员作为氨基酸转运体的主要组成部分,肿瘤细胞对于氨基酸的大量摄取主要是通过各种过表达的氨基酸转运体实现的 [7-8]。因此,通过靶向SLC家族的氨基酸转运体,实现对肿瘤生长的抑制成为一个有效的策略。

图1. 溶质转运体SLC介导的物质转运 [6-8]
2. 什么是SLC7A11?
溶质载体家族成员SLC7A11(Solute Carrier Family 7 Member 11,又名xCT)是溶质转运第7家族的第11个成员,属于胱氨酸/谷氨酸逆向转运蛋白,主要参与氨基酸在质膜上的转运 [9, 10]。SLC7A11基因位于人染色体4q28-q32,具有14个外显子,编码氨基酸转运载体xCT,其蛋白由501个氨基酸组成,包括12个跨膜结构域,其N端和C端均存在于细胞质内 [9, 10, 11](图2)。SLC7A11作为轻链亚基,与重链亚基SLC3A2组成胱氨酸/谷氨酸反转运体(Xc-系统),但发挥主要转运功能活性的是SLC7A11 [9-12]。近几年的国内外研究表明,SLC7A11高表达于多种实体恶性肿瘤,如乳腺癌、胰腺癌、卵巢癌和胶质瘤等等,且与恶性肿瘤的治疗耐药性有着密切的关系 [13-14]。目前,SLC7A11已经成为抗癌治疗的一个研究重点。
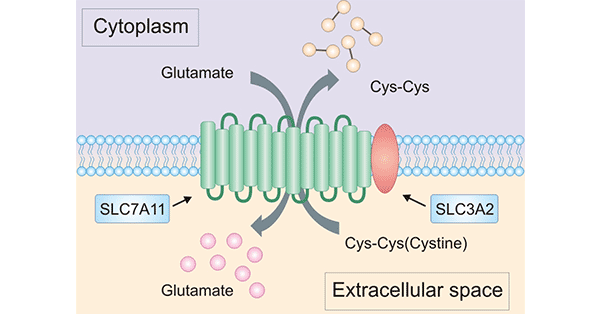
图2. SLC7A11的结构 [11]
3. SLC7A11相关的调节机制
3.1 SLC7A11和Xc-系统
胱氨酸/谷氨酸转运体系统(cystine/glutamate transporter, Xc-系统)是氨基酸转运体家族中的重要亚型。Xc-系统由轻链SLC7A11和重链SLC3A2两种蛋白质组成 [10]。SLC3A2作为其伴侣蛋白,用于维持SLC7A11蛋白的稳定性并调节SLC7A11向质膜的运输。轻链亚基SLC7A11作为Xc-系统的功能亚基,SLC7A11对胱氨酸和谷氨酸具有高度特异性,其作用是参与胱氨酸的胞外摄取和谷氨酸释放,促进谷胱甘肽(glutathione,r-glutamyl cysteingl+glycine,GSH)的合成,保护细胞免受氧化应激的损伤,维持细胞氧化还原平衡,从而阻止细胞因脂质过氧化而导致的细胞死亡 [9-12]。目前,对Xc-系统的研究主要集中于轻链亚基SLC7A11(图3) [41]。
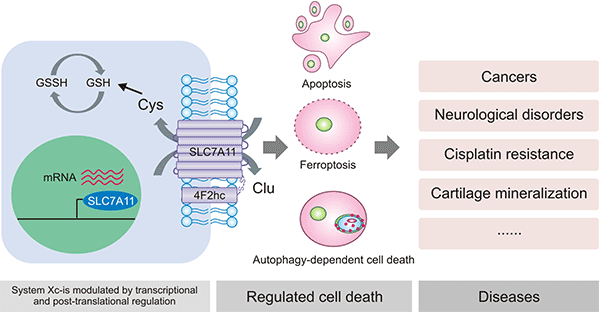
图3. SLC7A11 在Xc-系统中发挥主要作用 [41]
3.2 SLC7A11与铁死亡
SLC7A11是特异性的氨基酸转运蛋白,也是铁死亡的关键调节蛋白(点击可查看“铁死亡”专题文章)。SLC7A11的下调可通过抑制半胱氨酸代谢通路,导致细胞内胱氨酸水平降低和GSH生物合成耗竭,间接抑制GPX4的活性,进而导致脂质过氧化物堆积,最终诱导细胞发生铁死亡(图4) [15, 16]。此外,经典的铁死亡促进剂Erastin,可靶向SLC7A11,诱导铁死亡,逆转结直肠癌耐药 [17, 18]。研究人员发现,过表达SOCS2可促进铁死亡关键蛋白SLC7A11发生K48链型泛素化降解,调控肝癌中铁死亡的发生 [19]。近期还有研究表明,活化后的肿瘤蛋白53(p53)可以结合到SLC7A11基因的启动子区,从而抑制了SLC7A11基因的转录活性,影响GSH的合成,可诱导发生铁死亡 [20, 21]。
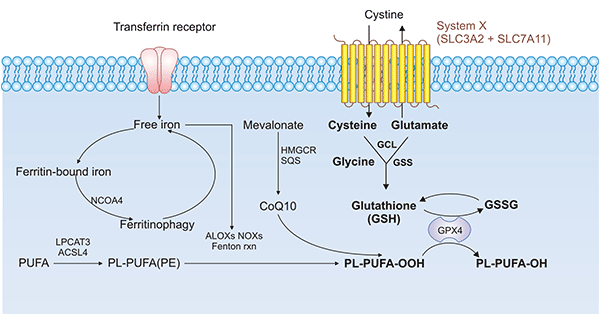
图4. SLC7A11/GPX4通路在铁死亡中的作用方式 [15, 16]
3.3 SLC7A11与肿瘤耐药
肿瘤中SLC7A11介导的GSH水平升高,其过程参与化疗药物治疗耐药。例如,在胶质瘤中,SLC7A11过表达,可增加胶质瘤细胞对氧化应激的抵抗力,降低对替莫唑胺(Temozolomide)的敏感性 [22, 23]。在黑色素瘤中,SLC7A11通过增加细胞内GSH含量,使黑色素瘤对BRAF抑制剂产生耐药性,而组蛋白去乙酰化酶抑制剂抑制SLC7A11能够显著诱导肿瘤消退 [25]。在大肠癌中,特异性SLC7A11抑制剂-柳氮磺吡啶(Sulfasalazine,SSZ),可有效增强顺铂(Cisplatin)在癌细胞内的药物含量和细胞毒性 [12]。在膀胱癌中,下调SLC7A11表达,耐药细胞对cisplatin的敏感性明显增加 [25]。三阴性乳腺癌患者中,SLC7A11、ATF4的表达远高于正常乳腺组织,eIF2α/ATF4轴上调SLC7A11的表达,促进GSH合成,抑制活性氧自由基ROS的积累,而eIF2α去磷酸化使SLC7A11表达降低,caspase-3表达增加,诱导三阴性乳腺癌细胞凋亡,使得对顺铂和多柔比星(Doxorubicin)更加敏感,降低耐药性 [26, 27]。因此,SLC7A11可能为治疗多种耐药相关肿瘤的治疗靶点。
4. SLC7A11在肿瘤靶向治疗中的作用
4.1 SLC7A11与神经系统肿瘤
研究表明,SLC7A11可作为转录激活因子4(ATF4)的靶标,在人胶质母细胞瘤细胞U87和U251中,过表达ATF4通过增加SLC7A11的表达,抑制肿瘤细胞发生铁死亡,促进血管生成,促进胶质母细胞瘤的增殖 [28, 29]。另有研究证实,通过敲低胶质母细胞瘤细胞的SLC7A11表达,或采用Nutlin-3a释放出p53负性调节SLC7A11,细胞中脂氧合酶ALOXE3的活性增加,促进胶质母细胞瘤铁死亡,抑制小鼠原位肿瘤的生长和迁移 [30]。构建SLC7A11基因敲除及过表达的U251胶质瘤细胞发现,SLC7A11基因敲除可增加ROS水平,降低GSH水平,促进细胞死亡;SLC7A11过表达可增加癌细胞对抗氧化应激,对替莫唑胺(Temozolomide)的敏感性减弱 [29]。
4.2 SLC7A11与乳腺癌及生殖系统肿瘤
据报道,IGF-I可激活雌激素受体阳性(estrogen receptor-positive,ER+)乳腺癌细胞中SLC7A11的表达,调节胱氨酸摄取和细胞氧化还原状态,促进ER+乳腺癌细胞增殖,该过程可被柳氮磺吡啶(SSZ)抑制 [31];一种针对SLC7A11的病毒样颗粒(virus-like-particle,VLP)AX09-0M6,采用VLP免疫方法抑制乳腺癌移植瘤小鼠的SLC7A11活性后,研究人员发现AX09-0M6对乳腺癌的生长和肺转移有明显抑制作用;采用牛疱疹病毒4型(BoHV-4)载体的抗SLC7A11病毒疫苗,它能够向体内传递细胞并赋予肿瘤抗原免疫原性,靶向作用于乳腺CSCs,抑制乳腺癌进展和转移,这种疫苗或可作为预防乳腺癌复发的潜在选择 [32]。在卵巢癌中,奥拉帕利(Olaparib)以p53依赖的方式,降低SLC7A11蛋白的表达水平,促进铁死亡,抑制肿瘤进展 [33]。
4.3 SLC7A11与呼吸系统肿瘤
多种肺癌亚型中SLC7A11均有表达。在肺癌中,铁死亡诱导剂Erastin,可诱导肺癌细胞铁死亡,随药物浓度的增高,细胞死亡率也增高 [34]。在KRAS突变的肺腺癌中,SLC7A11抑制剂选择性地增加了含有KRAS突变的肺腺癌的代谢应激和氧化应激介导的细胞死亡 [35]。另有报道,SLC7A11通过增强GSH合成,而对KRAS诱导的致瘤性至关重要 [10]。此外,SSZ通过抑制Xc-系统,可降低小细胞肺癌细胞内GSH水平,抑制癌细胞生长。在人喉鳞状细胞癌中,SLC7A1敲低,显著抑制肿瘤细胞G1至S相转变,证实下调SLC7A11,诱导G1相细胞周期停滞,抑制细胞增殖,表明SLC7A11可能是人喉鳞状细胞癌诊断和预后的重要生物标志物 [36]。
4.5 SLC7A11与消化系统肿瘤
在胃癌中,敲低MGC380细胞的生长分化因子15(GDF-15),导致SLC7A11表达下调,也促进Erastin诱导的胃癌细胞铁死亡,但不影响其它铁死亡相关基因,如GPX4、转铁蛋白、铁调素(Hepcidin)等 [37]。在胃癌小鼠模型中,采用SSZ抑制SLC7A11或对CD44基因切除,均可抑制胃癌细胞的生长。在吉西他滨(Gemcitabine)耐药的胰腺癌细胞中,SLC7A11表达上调,采用SSZ抑制SLC7A11与吉西他滨联合,抑制人胰腺癌细胞系PANC-1免疫缺陷小鼠异种移植瘤的生长,并增加肿瘤对Gemcitabine的敏感性。因此,基于靶向SLC7A11抑制剂的联合用药,有助于逆转胰腺癌细胞的Gemcitabine耐药 [10, 39, 40]。
5. SLC7A11的临床应用前景
来自Pharmsnap的数据显示,已有1款靶向SLC7A11抗体在研临床药物(Anti-xCT antibody-drug conjugate (Agilvax))处于临床前,用于肿瘤治疗。目前,SLC7A11已被广泛赋予各种癌症类型的化疗耐药性。多项研究证实,靶向SLC7A11可以逆转恶性肿瘤治疗过程中的耐药性。因此,SLC7A11抑制剂可以与临床一线化疗药物进行联合使用,以达到作用更持久、靶向性更强、不良反应更低的抗肿瘤作用。此外,SLC7A11广泛分布于多种恶性肿瘤中,在恶性肿瘤代谢调控中的作用提示,SLC7A11可作为肿瘤治疗的潜在靶点。
为鼎力协助各药企针对SLC7A11在各种肿瘤等疾病在临床中的研究,CUSABIO推出SLC7A11蛋白产品,跨膜次数高达12次(Code:CSB-CF892171HU(A4)),助力您在SLC7A11机制方面的研究或其潜在临床价值的探索。
Recombinant Human SLC7A11 (CSB-CF892171HU(A4))
● SDS Assay & High Specificity Validated by Western Blot
Recombinant Human Cystine/glutamate transporter(SLC7A11)
Code: CSB-CF892171HU(A4)
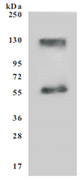
Recombinant Human Cystine/glutamate transporter(SLC7A11)
Code: CSB-CF892171HU(A4)
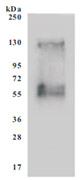
Recombinant Human Cystine/glutamate transporter(SLC7A11)
Code: CSB-CF892171HU(A4)
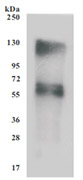
Recombinant Human Cystine/glutamate transporter(SLC7A11)
Code: CSB-CF892171HU(A4)
● Excellent Bioactivity Validated by Functional ELISA Blot
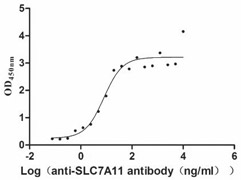
Recombinant Human Cystine/glutamate transporter(SLC7A11)
Code: CSB-CF892171HU(A4)
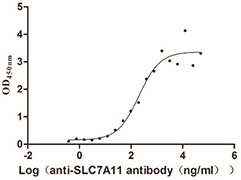
Recombinant Human Cystine/glutamate transporter(SLC7A11)
Code: CSB-CF892171HU(A4)
参考文献:
[1] Stockwell, Brent R. "Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications." Cell 185.14 (2022): 2401- 2421.
[2] Liu, Xiaodong. "SLC family transporters. "Drug Transporters in Drug Disposition, Effects and Toxicity (2019): 101-202.
[3] Rives, Marie-Laure, Jonathan A. Javitch, and Alan D. Wickenden. "Potentiating SLC transporter activity: emerging drug discovery opportunities." Biochemical pharmacology 135 (2017): 1-11.
[4] Mariem, O. Ben, et al. "Deciphering the ligand/xenobiotic molecular recognition mechanism in SLC transporters via a new structural binding site analysis." (2021).
[5] Li, Qiao, et al. "Subcellular drug distribution: mechanisms and roles in drug efficacy, toxicity, resistance, and targeted delivery." Drug Metabolism Reviews 50.4 (2018): 430-447.
[6] Liu, Xiaodong. "Transporter-mediated drug-drug interactions and their significance. "Drug Transporters in Drug Disposition, Effects and Toxicity ( 2019): 241-291.
[7] Zhou, Shiwei, and Yan Shu. "Transcriptional Regulation of Solute Carrier Drug Transporters." Drug Metabolism and Disposition 50.9 (2022): 1238-1250.
[8] César-Razquin, Adrián, et al. "A call for systematic research on solute carriers." cell 162.3 (2015): 478-487.
[9] Bassi, Maria, et al. "Identification and characterisation of human xCT that co-expresses, with 4F2 heavy chain, the amino acid transport activity system xc-." Pflügers Archiv 442.2 (2001): 286-296.
[10] Lin, Wenyu, et al. "SLC7A11/xCT in cancer: biological functions and therapeutic implications." American journal of cancer research 10.10 (2020): 3106.
[11] Koppula, Pranavi, et al. "Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer." Cancer Communications 38.1 (2018): 1-13.
[12] He, Jiaqin, et al. "The amino acid transporter SLC7A11-mediated crosstalk implicated in cancer therapy and the tumor microenvironment." Biochemical Pharmacology (2022): 115241.
[13] Li, Sijia, et al. "The Role of SLC7A11 in Cancer: Friend or Foe?" Cancers 14.13 (2022): 3059.
[14] Lin, Yi, et al. "Pan-Cancer Analyses Confirmed the Ferroptosis-Related Gene SLC7A11 as a Prognostic Biomarker for Cancer." International Journal of General Medicine 15 (2022): 2501.
[15] Koppula, Pranavi, Li Zhuang, and Boyi Gan. "Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy." Protein & cell 12.8 (2021): 599-620.
[16] Iida, Yuko, et al. "Effective ferroptotic small-cell lung cancer cell death from SLC7A11 inhibition by sulforaphane." Oncology letters 21.1 (2021): 1- 1.
[17] Xu, Xiaotian, et al. "Targeting SLC7A11 specifically suppresses the progression of colorectal cancer stem cells via inducing ferroptosis." European Journal of Pharmaceutical Sciences 152 (2020): 105450.
[18] Chen, Liang, et al. "GDF15 knockdown promotes erastin-induced ferroptosis by decreasing SLC7A11 expression." Biochemical and Biophysical Research Communications 526.2 (2020): 293-299.
[19] Chen, Qianping, et al. "SOCS2-enhanced ubiquitination of SLC7A11 promotes ferroptosis and radiosensitization in hepatocellular carcinoma." Cell Death & Differentiation (2022): 1-15.
[20] Guan, Zhenhua, et al. "Tanshinone IIA induces ferroptosis in gastric cancer cells through p53-mediated SLC7A11 down-regulation." Bioscience reports 40.8 (2020).
[21] Luo, Yi, et al. "Bavachin induces ferroptosis through the STAT3/P53/SLC7A11 axis in osteosarcoma cells." Oxidative medicine and cellular longevity 2021 (2021).
[22] Sehm, Tina, et al. "Temozolomide toxicity operates in a xCT/SLC7a11 dependent manner and is fostered by ferroptosis." oncotarget 7.46 (2016): 74630.
[23] Hu, Zhifang, et al. "A potential mechanism of temozolomide resistance in glioma-ferroptosis." Frontiers in oncology 10 (2020): 897.
[24] An, Lifeng, et al. "lncRNA AGAP2-AS1 Facilitates Tumorigenesis and Ferroptosis Resistance through SLC7A11 by IGF2BP2 Pathway in Melanoma." Computational and Mathematical Methods in Medicine 2022 (2022).
[25] Drayton, Ross M., et al. "Reduced Expression of miRNA-27a Modulates Cisplatin Resistance in Bladder Cancer by Targeting the Cystine/Glutamate Exchanger SLC7A11Cisplatin Resistance in Bladder Cancer." clinical cancer research 20.7 (2014): 1990-2000.
[26] Yadav, Poonam, et al. "SLC7A11/xCT is a target of miR-5096 and its restoration partially rescues miR-5096-mediated ferroptosis and anti-tumor effects in human breast cancer cells." Cancer Letters 522 (2021): 211-224.
[27] Sun, Chen, et al. "Propofol Inhibits Proliferation and Augments the Anti-Tumor Effect of Doxorubicin and Paclitaxel Partly Through Promoting Ferroptosis in Triple-Negative Breast Cancer Cells." Frontiers in Oncology 12 (2022): 837974-837974.
[28] Polewski, Monika D., et al. "SLC7A11 overexpression in glioblastoma is associated with increased cancer stem cell-like properties." Stem Cells and Development 26.17 (2017): 1236-1246.
[29] Polewski, Monika D., et al. "Increased expression of system xc- in glioblastoma confers an altered metabolic state and temozolomide resistance." Molecular Cancer Research 14.12 (2016): 1229-1242.
[30] Yang, Xinzhi, et al. "miR-18a promotes glioblastoma development by down-regulating ALOXE3-mediated ferroptotic and anti-migration activities." Oncogenesis 10.2 (2021): 1-13.
[31] Yang, Y., M. A. Becker, and D. Yee. "P2-03-03: An Insulin-Like Growth Factor I (IGF-I)-Induced Gene, Solute Carrier Family 7 Member 11 (SLC7A11)/xCT, Mediates IGF-I-Induced Biological Behaviors in Breast Cancer Cells." Cancer Research 71.24_Supplement (2011): P2-03.
[32] Wang, Zhanyu, Qianjin Jiang, and Chenfang Dong. "Metabolic reprogramming in triple-negative breast cancer. "Cancer Biology & Medicine 17.1 (2020 ): 44.
[33] Hong, Ting, et al. "PARP inhibition promotes ferroptosis via repressing SLC7A11 and synergizes with ferroptosis inducers in BRCA-proficient ovarian cancer." Redox biology 42 (2021): 101928.
[34] Huang, Chaoli, et al. "Upregulation and activation of p53 by erastin-induced reactive oxygen species contribute to cytotoxic and cytostatic effects in A549 lung cancer cells." Oncology reports 40.4 (2018): 2363-2370.
[35] Hu, Kewen, et al. "Suppression of the SLC7A11/glutathione axis causes synthetic lethality in KRAS-mutant lung adenocarcinoma." the Journal of clinical investigation 130.4 (2020): 1752-1766.
[36] Ma, Zhihong, et al. "SLC7A11, a component of cysteine/glutamate transporter, is a novel biomarker for the diagnosis and prognosis in laryngeal squamous cell carcinoma." Oncology Reports 38.5 (2017): 3019-3029.
[37] Chen, Liang, et al. "GDF15 knockdown promotes erastin-induced ferroptosis by decreasing SLC7A11 expression." Biochemical and Biophysical Research Communications 526.2 (2020): 293-299.
[38] Toyoshima, Kosei, et al. "Analysis of circulating tumor cells derived from advanced gastric cancer." international journal of cancer 137.4 (2015): 991 -998.
[39] Tang, Rong, et al. "The role of ferroptosis regulators in the prognosis, immune activity and gemcitabine resistance of pancreatic cancer." Annals of translational medicine 8.21 (2020).
[40] Tang, Rong, et al. "The role of ferroptosis regulators in the prognosis, immune activity and gemcitabine resistance of pancreatic cancer." Annals of translational medicine 8.21 (2020).
[41] Tu, H., et al. "Insights into the novel function of system Xc-in regulated cell death." Eur Rev Med Pharmacol Sci 25.3 (2021): 1650-1662.



