武汉华美生物工程有限公司CUSABIO®品牌商
15 年
手机商铺
- NaN
- 0
- 0
- 2
- 2
推荐产品
公司新闻/正文
MUC17:粘蛋白“冷”靶点,探索胃癌新疗法!
777 人阅读发布时间:2023-01-04 09:58
近来越来越多的研究证实,粘蛋白多样化的生物学功能在肿瘤形成、细胞粘附、免疫应答以及细胞信号传导中起着至关重要的作用。当前,多个粘蛋白已成为肿瘤免疫治疗的火热靶点,如MUC1,MUC16。此外,根据ClinicalTrials数据显示,新近一项基于MUC17靶点(AMG199)的双抗临床项目,正在美国开展I期研究(NCT04117958),用于转移性胃癌和胃食管交界癌(G/GEJ)患者,这是第一个将MUC17作为潜在抗肿瘤靶点的临床试验。
在肿瘤组织中,粘蛋白家族靶点多出现异常表达,与肿瘤的浸润、转移及预后相关。近年来,多个粘蛋白被认为是某些癌症的重要生物标志物,以及非常具有前景的治疗靶点。相比其它火热的粘蛋白靶点,MUC17作为粘蛋白“冷”靶点,虽尚未经广泛推广、暂无药物上市,然而陆续的研究表明,MUC17是潜力非常不错的免疫靶点!或许能为胃癌和胃食管交界癌(G/GEJ)或其它疾病治疗带来新曙光!
1. 什么是粘蛋白(Mucin)?
2. 什么是MUC17?
3. MUC17介导的信号转导通路
4. MUC17在肿瘤中的作用
5. MUC17的临床应用前景
1. 什么是粘蛋白(Mucin)?
粘蛋白(Mucin,MUC)是上皮细胞产生、分泌的以粘液为主要成分的高分子糖蛋白。迄今为止,已发现有22种Mucin蛋白,根据黏蛋白的功能将其分为两类,膜结合型粘蛋白(MUC1、MUC3A、MUC3B、MUC4、MUC12、MUC13、MUC14、MUC15、MUC16、MUC17、MUC20、MUC21、MUC22)和分泌型粘蛋白(MUC2、MUC5AC、MUC5B、MUC6、MUC7、MUC8、MUC9、MUC19) [1-3]。分泌型粘蛋白可以形成物理屏障,作为一种黏液凝胶,为呼吸道和胃肠道上皮细胞提供保护。跨膜型粘蛋白通过它们胞外域的O糖基化串联重复序列形成棒状结构,构建保护性的黏液凝胶层 [3]。大量炎症细胞因子,比如白介素-1β(interleukin-1β,IL-1β)、IL-4、IL-6、TGF-β、IL-9、IL-13、IFN-γ和TNF-α,已经被证明能促进体外培养的上皮细胞内黏蛋白的表达 [4-5]。
黏蛋白广泛分布于机体多种器官和组织的黏膜表面,如消化道、呼吸道、生殖道、角结膜,以及肝脏、胰腺、胆囊、肾、唾液腺和泪腺上皮细胞 [3-4]。近年发现黏蛋白参与上皮细胞的分化、更新、调节细胞黏附、细胞信号转导等过程 [1]。有研究提示,粘蛋白可作为预测COVID-19易感性的潜在工具 [5]。此外,大量研究已证实,黏蛋白基因在肿瘤的发生发展中发挥重要作用,且因其具有组织特异性和细胞特异性,目前越来越受到国内外学者的重视(图1)[6-10]。

图1. 黏蛋白在肿瘤发生发展中发挥重要作用 [6]
2. 什么是MUC17?
MUC17(Mucin-17,又称MUC3)是一种跨膜粘蛋白,由位于7号染色体的q22位点的MUC17基因编码。MUC17于2002年发现并被鉴定,由跨膜段、胞内段和胞外段三部分组成(图2) [11, 14]。从氨基末端依次为粘蛋白样结构(串联重复序列),EGF(表皮生长因子)样区域,N-糖基化区域,第二个EGF样区域,疏水的跨膜段,及胞浆内部的梭基末端 [12]。MUC17依靠羧基端附近跨膜区域与细胞膜结合,可参与细胞内信号传递。胞外段决定着MUC特异性的空间结构和免疫原性 [14]。MUC17的表达可增强肠道粘液屏障,促进炎症愈合。在上皮细胞中,MUC17起到参与信号转导,维持管腔结构,提供细胞保护,并给予失去极性的癌细胞抗黏附性能,从而使肿瘤细胞失去极性 [15]。
MUC17主要表达于肠道,是肠道黏液的主要组成成分。MUC17表达可在胎儿小肠和结肠内被检测。多数研究表明,正常胃粘膜无表达或少表达 [16-18]。研究发现,从正常胃黏膜、肠化黏膜、进展期胃癌的发展过程中,MUC17的表达逐渐明显增高,其低表达与胃癌预后不良相关。进一步研究显示MUC17的表达与肿瘤的分化程度呈负相关,提示MUC17可作为胃癌的诊断和治疗靶标 [19]。目前,基于MUC17的双抗正在进行临床研究,用于阳性转移性胃癌和胃食管交界癌患者 [20]。

图2. MUC17的结构 [14]
3. MUC17介导的信号转导通路
MUC17作为具重要生物学功能的膜结合型粘蛋白,根据国内外文献报道,MUC17表达水平与多种炎症、肿瘤细胞增殖相关,这为MUC17相关性疾病的防治提供了重要线索,但其分子机制不清。
在结肠腺癌细胞中,用促炎性细胞因子TNFα的长期刺激,诱导炎性状态,可导致MUC17的增加,进而保护结肠腺癌细胞免受肠病原性大肠杆菌的黏附,其结果说明MUC17在炎症中可起到防御作用(图3)[21]。另有研究提示,MUC17的EGF样结构域可通过与MYH9相互作用,活化Rho/Rock通路,从而抑制炎症因子对NFκB通路的活化,最终致使MUC17发挥了其抗炎的功能 [22]。还有一些关于MUC17的报道揭示,MUC17的C端与支架蛋白PDZK1结合,使其稳定定位于小肠的肠细胞顶膜 [23];DNA甲基化和组蛋白H3K9修饰有助于MUC17表达 [24]。
同样在结肠癌中,有研究揭示MUC17可与EGFR家族的ERBB2结合并使其磷酸化,激活Wnt信号,调控EMT靶基因表达进而启动EMT [25-26]。此外,还有研究表明,初乳来源的、含TFLK基序的合成肽,能刺激结肠细胞株HT29细胞MUC17表达增加。TFLK基序是MSAA3蛋白的特征结构序列,而MSAA3蛋白对新生儿预防病原菌侵袭、保护新生儿胃肠道有重要作用。该机制对增强肠道粘液屏障,防治肠道多种炎症性疾病将提供新的思路 [27-28]。
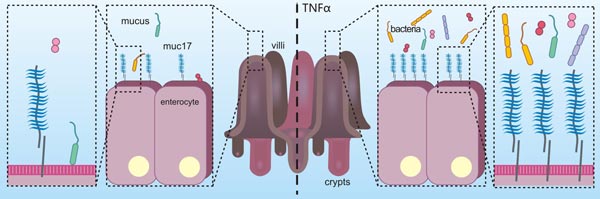
图3. MUC17在炎症中可起到防御作用 [21]
4. MUC17在肿瘤中的作用
MUC17作为一个典型的跨膜黏蛋白,MUC17在抵御细菌侵袭、保护肠黏膜、促进上皮增生及炎症愈合中发挥着重要作用,其表达减少是多种肠道疾病如新生儿肠炎、炎症性肠病发生发展的重要原因 [29, 30]。目前,大量的研究集中在MUC17与胃肠道肿瘤和其它肿瘤,如结肠癌、胃癌、胰腺癌、食管癌、乳腺癌 [31-40]。
在结肠癌中,采用免疫组织化学法,检测MUC17在结直肠癌及癌旁相对正常黏膜组织中的表达,MUC17在正常黏膜组织也呈100%阳性表达,结直肠癌标本中MUC17阳性表达率为53.3%,表达呈下调趋势,且MUC17的表达与结直肠癌的淋巴结转移、浸润深度和分期密切相关 [16, 31]。
在胰腺癌中,与正常胰腺组织比较,MUC17在胰腺癌组织中过表达,用免疫组化检测胰腺导管腺癌组织发现,MUC17表达显著增强,提示MUC17可作为独立的预后因素之一 [32-33]。另有研究提示,MUC17的表达变化与Barrett食管、食管腺癌、贲门肠化和贲门癌的发展呈正相关 [35-37]。在乳腺癌中,研究发现MUC17敲低与患者药物敏感性有关,进一步揭示MUC17和PCNX1可作为乳腺癌化疗反应的潜在生物标志物,调节其活性可以影响化疗耐药性 [25, 38-39]。
在胃癌及胃食管交界处癌(G/GEJ)中,有研究证实MUC17在G/GEJ细胞膜上过表达,属于肿瘤相关抗原(TAA)。胃癌与胃粘膜屏障功能障碍有关,而胃粘膜屏障的损害通常会导致慢性炎症,这是胃癌发生的主要因素。进一步研究发现,当MUC17表达降低,肿瘤侵袭细胞个数减少,细胞划痕愈合率及细胞增殖率降低,克隆形成能力减弱。敲低MUC17表达可抑制胃癌细胞的侵袭和增殖能力,这提示MUC17或将可能成为胃癌治疗的新靶标 [40]。
5. MUC17的临床应用前景
目前已有一款靶向MUC17的双特异性抗体(AMG199;MUC17 x CD3)获临床许可,来自安进生物,用于MUC17阳性的实体肿瘤,包括胃肠癌、胃食管交界处、结肠直肠癌和胰腺癌。2020年,FDA授予AMG199孤儿药资格认定。2021年ASCO大会上,安进公布了首次在人体的I期开放标签剂量递增研究,评估AMG199治疗MUC17阳性胃/胃食管交界癌症患者的剂量限制性毒性及有效率,并确定最大耐受剂量(MTD)和/或推荐的2期剂量(RP2D),预计在2024年3月完成70名受试者临床I期研究。
大量研究提示粘蛋白在不同恶性肿瘤中过表达,粘蛋白与肿瘤的密切关系及诱发的免疫反应为基于粘蛋白的抗肿瘤药物设计提供了重要线索,许多新突破,新的药物、新的治疗方案正在路上。与此同时,随着对粘蛋白MUC17结构和功能研究的进一步深入,MUC17在肿瘤中的研究展示了良好的应用前景,我们也期待MUC17靶点在肿瘤免疫治疗方面带来创新性或突破性进展!
为鼎力协助各药企针对MUC17在肿瘤等疾病治疗领域上的研发,CUSABIO推出MUC17活性蛋白产品(Code: CSB-MP727848HU,助力您在MUC17机制方面的研究或其潜在临床价值的探索。
Recombinant Human Mucin-17(MUC17), partial (Active)
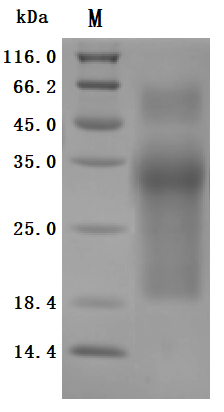
The purity was greater than 95% as determined by SDS-PAGE. (Tris-Glycine gel) Discontinuous SDS-PAGE (reduced) with 5% enrichment gel and 15% separation gel.
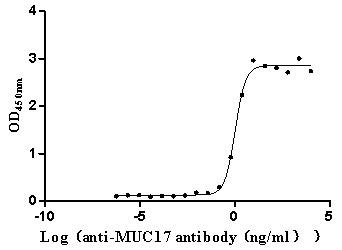
Immobilized Human MUC17 at 2 μg/mL can bind Anti-MUC17 recombinant antibody (CSB-RA727848MA1HU), the EC50 is 0.9057-1.259 ng/mL.
参考文献:
[1] Dekker, Jan, et al. "The MUC family: an obituary." Trends in biochemical sciences 27.3 (2002): 126-131.
[2] Alcântara, Angélica Leite de, et al. "MUC family influence on acute lymphoblastic leukemia in Native American populations from Brazilian Amazon." (2022): e19025-e19025.
[3] Sousa, Andreia M., et al. "Reflections on MUC 1 glycoprotein: the hidden potential of isoforms in carcinogenesis." Apmis 124.11 (2016): 913-924.
[4] Długosz, Ewa, et al. "Toxocara canis mucins among other excretory-secretory antigens induce in vitro secretion of cytokines by mouse splenocytes." Parasitology research 114.9 (2015): 3365-3371.
[5] Lin, Susanne Je-Han, Bailey Arruda, and Eric Burrough. "Alteration of Colonic Mucin Composition and Cytokine Expression in Acute Swine Dysentery." Veterinary Pathology 58.3 (2021): 531-541.
[6] Bhatia R, Gautam SK, Cannon A, et al. Cancer-associated mucins: role in immune modulation and metastasis. Cancer Metastasis Rev. 2019;38(1-2):223-236.
[7] Brockhausen, Inka, and Jacob Melamed. "Mucins as anti-cancer targets: Perspectives of the glycobiologist." Glycoconjugate Journal 38.4 (2021): 459-474.
[8] Marimuthu, Saravanakumar, et al. "Mucins reprogram stemness, metabolism and promote chemoresistance during cancer progression." Cancer and Metastasis Reviews 40.2 (2021): 575-588.
[9] Wi, Dong-Han, Jong-Ho Cha, and Youn-Sang Jung. "Mucin in cancer: a stealth cloak for cancer cells." BMB reports 54.7 (2021): 344.
[10] Kufe, Donald W. "Mucins in cancer: function, prognosis and therapy." Nature Reviews Cancer 9.12 (2009): 874-885.
[11] Shekels LL, Ho SB. Characterization of the mouse Muc3 membrane bound intestinal mucin 5' coding and promoter regions: regulation by inflammatory cytokines. Biochim Biophys Acta. 2003;1627(2-3):90-100.
[12] Desseyn, Jean-Luc, Daniel Tetaert, and Valérie Gouyer. "Architecture of the large membrane-bound mucins." Gene 410.2 (2008): 215-222.
[13] Gum Jr, James R., et al. "MUC17, a novel membrane-tethered mucin." Biochemical and biophysical research communications 291.3 (2002): 466-475.
[14] Moniaux, Nicolas, et al. "Characterization of human mucin MUC17: Complete coding sequence and organization." Journal of Biological Chemistry 281.33 (2006): 23676-23685.
[15] Gum Jr, James R., et al. "MUC17, a novel membrane-tethered mucin." Biochemical and biophysical research communications 291.3 (2002): 466-475.
[16] Senapati, Shantibhusan, et al. "Expression of intestinal MUC17 membrane-bound mucin in inflammatory and neoplastic diseases of the colon." Journal of clinical pathology 63.8 (2010): 702-707.
[17] Gál, Eleonóra, et al. "Importance of MUC17 in the Bile-Induced Pancreatic Cancer Progression." (2022).
[18] Layunta, Elena, et al. "IL-22 promotes the formation of a MUC17 glycocalyx barrier in the postnatal small intestine during weaning." Cell Reports 34.7 (2021): 108757.
[19] Bailis, Julie M., et al. "Preclinical evaluation of BiTE® immune therapy targeting MUC17 or CLDN18. 2 for gastric cancer." Cancer Research 80.16_Supplement (2020): 3364-3364.
[20] Lordick, F., et al. "P-76 A phase 1 study of AMG 199, a half-life extended bispecific T-cell engager (HLE BiTE®) immune therapy, targeting MUC17 in patients with gastric and gastroesophageal junction cancer." Annals of Oncology 31 (2020): S114.
[21] Schneider, Hannah, et al. "The human transmembrane mucin MUC17 responds to TNFα by increased presentation at the plasma membrane." Biochemical Journal 476.16 (2019): 2281-2295.
[22] Yang, Bing, et al. "Mucin 17 inhibits the progression of human gastric cancer by limiting inflammatory responses through a MYH9-p53-RhoA regulatory feedback loop." Journal of Experimental & Clinical Cancer Research 38.1 (2019): 1-13.
[23] Malmberg, Emily K., et al. "The C-terminus of the transmembrane mucin MUC17 binds to the scaffold protein PDZK1 that stably localizes it to the enterocyte apical membrane in the small intestine." Biochemical Journal 410.2 (2008): 283-289.
[24] Kitamoto, Sho, et al. "DNA methylation and histone H3-K9 modifications contribute to MUC17 expression." Glycobiology 21.2 (2011): 247-256.
[25] Yu, Kuan, et al. "Intratumoral PD-1+ CD8+ T cells associate poor clinical outcomes and adjuvant chemotherapeutic benefit in gastric cancer." British journal of cancer 127.9 (2022): 1709-1717.
[26] Al Amri, Waleed S., et al. "Genomic and Expression Analyses Define MUC17 and PCNX1 as Predictors of Chemotherapy Response in Breast CancerMUC17 and PCNX1 Predict Chemoresponse." Molecular cancer therapeutics 19.3 (2020): 945-955.
[27] Pan, Qiong, et al. "Enhanced Membrane-tethered Mucin 3 (MUC3) Expression by a Tetrameric Branched Peptide with a Conserved TFLK Motif Inhibits Bacteria Adherence*[S]." Journal of Biological Chemistry 288.8 (2013): 5407-5416.
[28] Qiong, P. A. N., et al. "Influence of MSAA3 protein fragment and modified peptide thereof on the MUC3 expression of HT29 cells." Medical Journal of Chinese People's Liberation Army 36.10 (2011): 1062-1064.
[29] Resta-Lenert, Silvia, et al. "Muc17 protects intestinal epithelial cells from enteroinvasive E. coli infection by promoting epithelial barrier integrity." American Journal of Physiology-Gastrointestinal and Liver Physiology 300.6 (2011): G1144-G1155.
[30] Yang, Ching-Wen, et al. "Genetic variations of MUC17 are associated with endometriosis development and related infertility." BMC medical genetics 16.1 (2015): 1-7.
[31] Jiang, Zhipeng, et al. "Analysis of TGCA data reveals genetic and epigenetic changes and biological function of MUC family genes in colorectal cancer." Future Oncology 15.35 (2019): 4031-4043.
[32] Kitamoto, Sho, et al. "Expression of MUC17 is regulated by HIF1α-mediated hypoxic responses and requires a methylation-free hypoxia responsible element in pancreatic cancer." (2012): e44108.
[33] Kitamoto, Sho, et al. "Expression of MUC17, a marker of pancreatic cancer, is under the control of epigenetic modifications." Cancer Research 71.8_Supplement (2011): 82-82.
[34] Hyland, Paula L., et al. "Global changes in gene expression of Barrett's esophagus compared to normal squamous esophagus and gastric cardia tissues." PLoS One 9.4 (2014): e93219.
[35] Ye et al. "The role of MUC1, MUC3 and MUC6 in Barrett's esophagus, esophageal adenocarcinoma, cardia intestinal metaplasia and cardia adenocarcinoma." 2006.
[36] Glickman, Jonathan N., Aliakbar Shahsafaei, and Robert D. Odze. "Mucin core peptide expression can help differentiate Barrett's esophagus from intestinal metaplasia of the stomach." The American journal of surgical pathology 27.10 (2003): 1357-1365.
[37] Lonie, James M., Andrew P. Barbour, and Riccardo Dolcetti. "Understanding the immuno-biology of oesophageal adenocarcinoma: Towards improved therapeutic approaches." Cancer Treatment Reviews 98 (2021): 102219.
[38] Rakha, Emad A., et al. "Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer." Modern Pathology 18.10 (2005): 1295-1304.
[39] Al Amri, W., et al. "Abstract P3-06-19: MUC17 and PCNX1 as mediators of chemotherapy response in breast cancer." Cancer Research 79.4_Supplement (2019): P3-06.
[40] Chao, Joseph, et al. "Trial in progress: A phase I study of AMG 199, a half-life extended bispecific T-cell engager (HLE BiTE) immune therapy, targeting MUC17 in patients with gastric and gastroesophageal junction (G/GEJ) cancer." (2020): TPS4649-TPS4649.



